Question: (14%) Problem 4: Suppose you want to raise the temperature of a 0.185-kg piece of ice from -20.0C to 130C. The heat of fusion
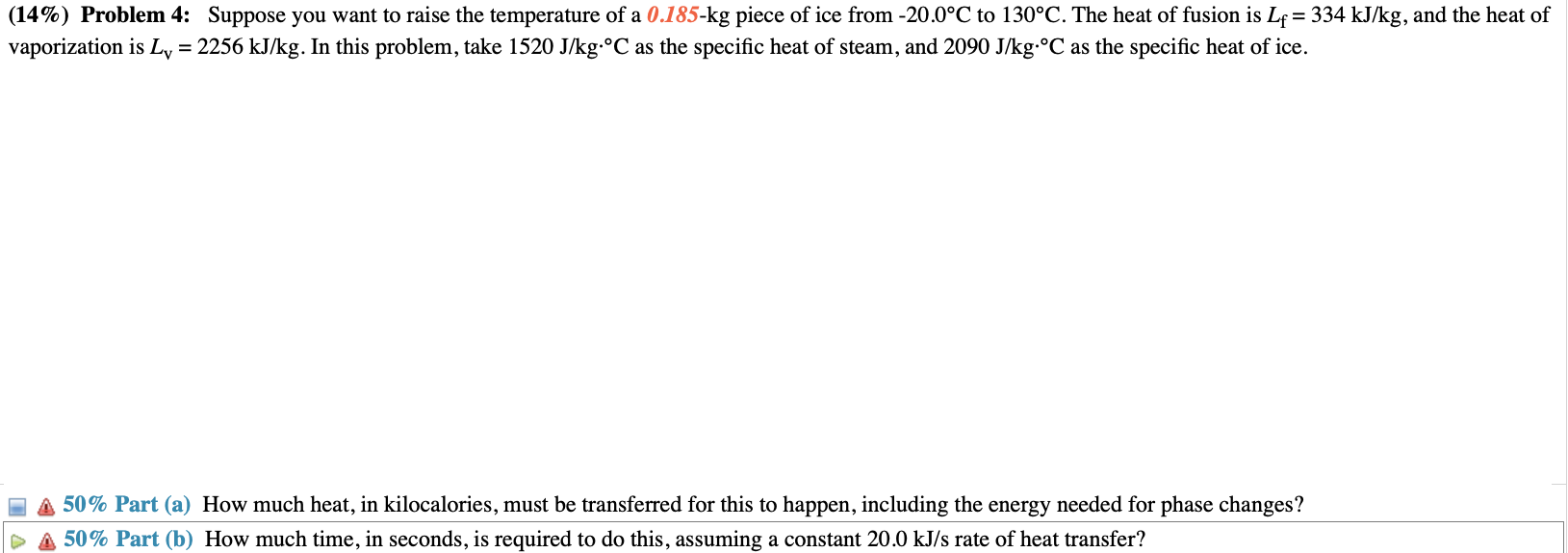
(14%) Problem 4: Suppose you want to raise the temperature of a 0.185-kg piece of ice from -20.0C to 130C. The heat of fusion is Lf = 334 kJ/kg, and the heat of vaporization is Ly = 2256 kJ/kg. In this problem, take 1520 J/kg C as the specific heat of steam, and 2090 J/kg C as the specific heat of ice. 50% Part (a) How much heat, in kilocalories, must be transferred for this to happen, including the energy needed for phase changes? 50% Part (b) How much time, in seconds, is required to do this, assuming a constant 20.0 kJ/s rate of heat transfer?
Step by Step Solution
There are 3 Steps involved in it
Part a Heat Required Well calculate the heat needed for each stage of the process and add them toget... View full answer

Get step-by-step solutions from verified subject matter experts


