Question: 15.128 When NO2 is bubbled into water, it disproportionates completely into HNO3 and HNO2 : 2NO2(g)+H2O(l)HNO3(aq)+HNO2(aq) Calculate the pH and the concentrations of all species
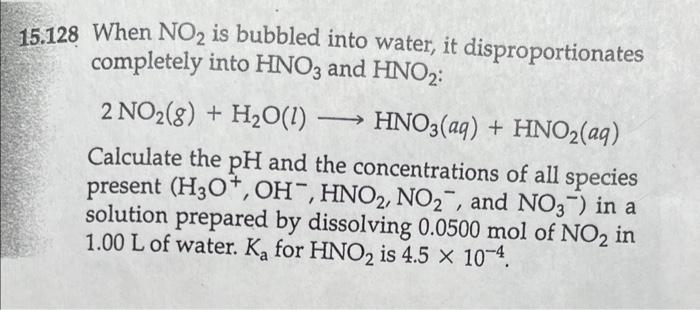
15.128 When NO2 is bubbled into water, it disproportionates completely into HNO3 and HNO2 : 2NO2(g)+H2O(l)HNO3(aq)+HNO2(aq) Calculate the pH and the concentrations of all species present (H3O+,OH,HNO2,NO2, and NO3)in a solution prepared by dissolving 0.0500mol of NO2 in 1.00L of water. Ka for HNO2 is 4.5104
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


