Question: SCH 401 EXERCISE: DETERMINING RATE Knowledge Nitric acid is an important industrial chemical with a variety of uses. It can be formed when nitrogen dioxide
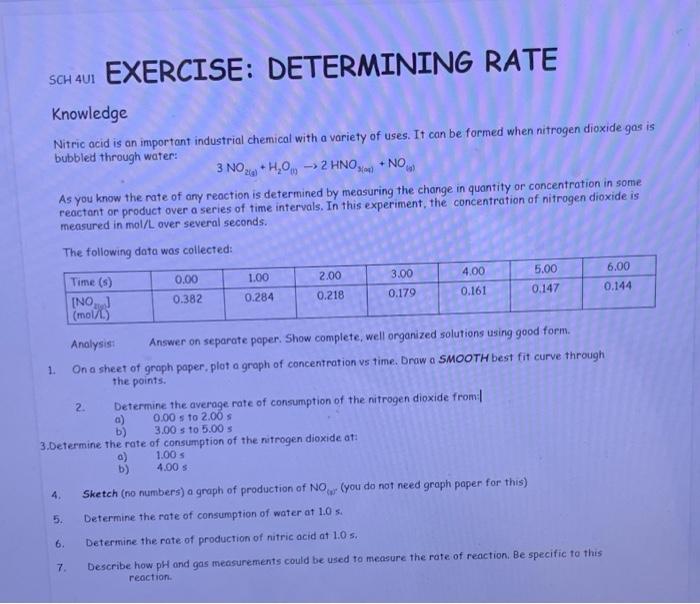
SCH 401 EXERCISE: DETERMINING RATE Knowledge Nitric acid is an important industrial chemical with a variety of uses. It can be formed when nitrogen dioxide gas is bubbled through water: 3 NO2+ H,0,. -> 2 HNO NO. As you know the rate of any reaction is determined by measuring the change in quantity or concentration in some reactant or product over a series of time intervals. In this experiment, the concentration of nitrogen dioxide is measured in mol/L over several seconds. The following data was collected: Time (s) 0.00 1.00 2.00 3.00 4.00 5.00 6.00 [NO] 0.382 0.284 0.218 0.179 0.161 0.147 0.144 (mol/l.) Analysis: Answer on separate paper. Show complete, well organized solutions using good form. 1 Ona sheet of graph paper. plot a graph of concentration vs time. Draw a SMOOTH best fit curve through the points 2. Determine the average rate of consumption of the nitrogen dioxide from a) 0.00 s to 2.00 b) 3.00 3 to 5.00 $ 3.Determine the rate of consumption of the nitrogen dioxide at: a) 1.00 5 b) 4.00 5 4. Sketch (no numbers) a graph of production of NO you do not need graph paper for this) 5. Determine the rate of consumption of water at 1.0 s. 6. Determine the rate of production of nitric acid at 1.0 s. 7 Describe how pH and gas measurements could be used to measure the rate of reaction. Be specific to this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


