Question: 16. Given H2SO4 + 2NH3 2NH4++ SO2, how many mL of 30.0 wt% H2SO4 will be required to completely react with 1.00 mol of
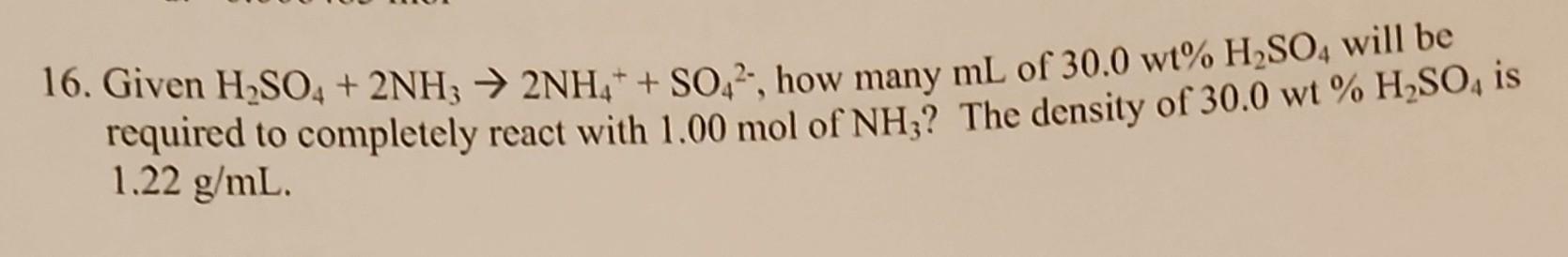
16. Given H2SO4 + 2NH3 2NH4++ SO2, how many mL of 30.0 wt% H2SO4 will be required to completely react with 1.00 mol of NH3? The density of 30.0 wt % H2SO4 is 1.22 g/mL.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


