Question: 18. (3 points) Element X has the following ionization energy values: 1 st ionization energy =578k//mol 2nd ionization energy =1820kJ/mol 3rd ionization energy
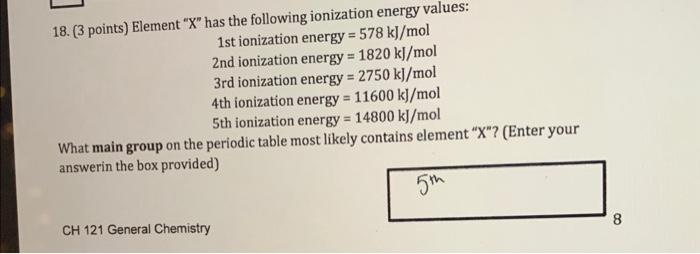
18. (3 points) Element " X " has the following ionization energy values: 1 st ionization energy =578k//mol 2nd ionization energy =1820kJ/mol 3rd ionization energy =2750kj/mol 4 th ionization energy =11600kJ/mol 5 th ionization energy =14800kJ/mol What main group on the periodic table most likely contains element " X "? (Enter your answerin the box provided) CH121 General Chemistry
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


