Question: 1.a 1.b 1.c Write the equation for the neutralization reaction in which potassium sulfide is the salt formed. Show the reaction in which the fully
1.a
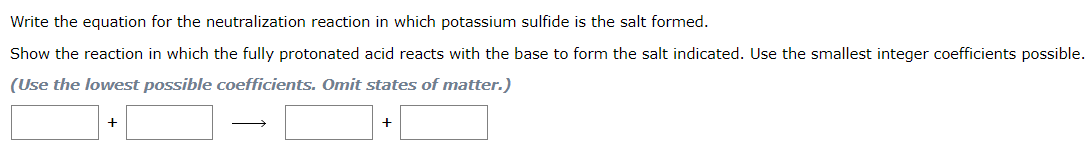 1.b
1.b
1.c

Write the equation for the neutralization reaction in which potassium sulfide is the salt formed. Show the reaction in which the fully protonated acid reacts with the base to form the salt indicated. Use the smallest integer coefficients possible. (Use the lowest possible coefficients. Omit states of matter.) ++ Write the equation for the reaction described: Solid lead(II) oxide reacts with solid carbon to produce lead metal and carbon dioxide. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) Silver chloride precipitates when solutions of sodium chloride and silver nitrate are combined. The precipitation of silver chloride. Write the equation for the reaction. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) +
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


