Question: 1B. (1) A batch adsorption study was performed to characterize the performance of an adsorbent (activated carbon) for the adsorption of an organic chemical
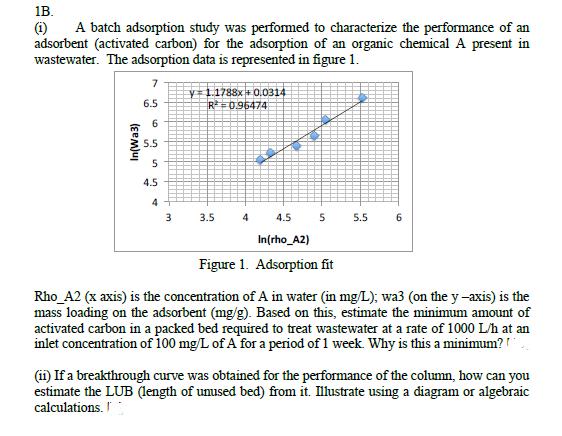
1B. (1) A batch adsorption study was performed to characterize the performance of an adsorbent (activated carbon) for the adsorption of an organic chemical A present in wastewater. The adsorption data is represented in figure 1. In(Wa3) 6.5 6 5.5 5 4.5 4 3 v=1.1788x + 0.0314 R=0.96474 3.5 4.5 In(rho_A2) Figure 1. Adsorption fit 4 5 5.5 6 Rho_A2 (x axis) is the concentration of A in water (in mg/L); wa3 (on the y -axis) is the mass loading on the adsorbent (mg/g). Based on this, estimate the minimum amount of activated carbon in a packed bed required to treat wastewater at a rate of 1000 L/h at an inlet concentration of 100 mg/L of A for a period of 1 week. Why is this a minimum?! (ii) If a breakthrough curve was obtained for the performance of the column, how can you estimate the LUB (length of unused bed) from it. Illustrate using a diagram or algebraic calculations.
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
i r 65 6 s st S 45 4 y 11788x 00314 R 036474 3 This a mini... View full answer

Get step-by-step solutions from verified subject matter experts


