Question: 2. (25 pts) Determine the coordination number and atomic packing factor for a simple cubic crystal structure. 3. (20 pts) Determine the theoretical density for


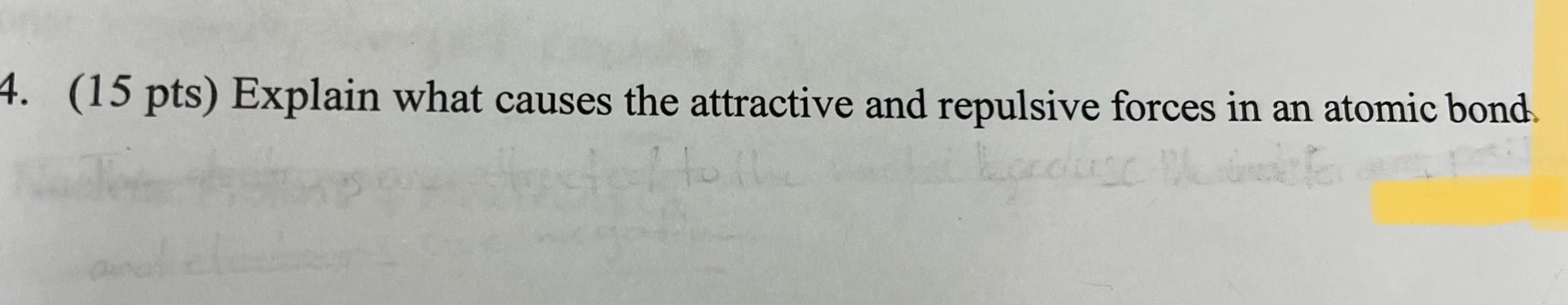
2. (25 pts) Determine the coordination number and atomic packing factor for a simple cubic crystal structure. 3. (20 pts) Determine the theoretical density for zinc, and compare it to its actual value. What accounts for the difference? 4. (15 pts) Explain what causes the attractive and repulsive forces in an atomic bond Groc
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


