Question: 2. (a) (b) (c) (d) (e) The van der Waals equation of state can be used to estimate any one of the state variables, P,
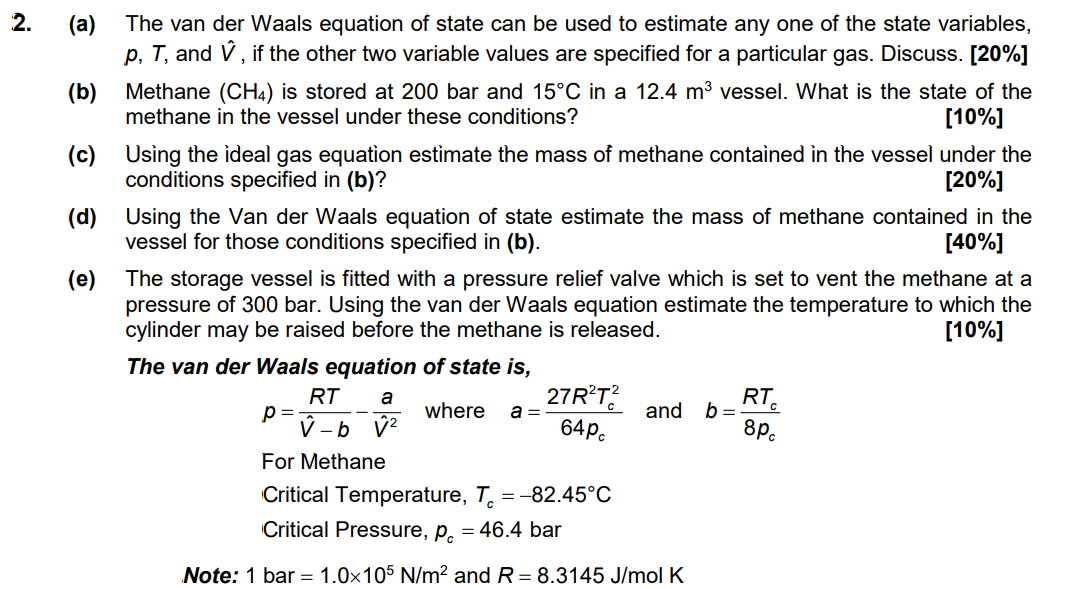
2. (a) (b) (c) (d) (e) The van der Waals equation of state can be used to estimate any one of the state variables, P, T, and , if the other two variable values are specified for a particular gas. Discuss. [20%] Methane (CH4) is stored at 200 bar and 15C in a 12.4 m vessel. What is the state of the methane in the vessel under these conditions? (10%] Using the ideal gas equation estimate the mass of methane contained in the vessel under the conditions specified in (b)? [20%] Using the Van der Waals equation of state estimate the mass of methane contained in the vessel for those conditions specified in (b). [40%] The storage vessel is fitted with a pressure relief valve which is set to vent the methane at a pressure of 300 bar. Using the van der Waals equation estimate the temperature to which the cylinder may be raised before the methane is released. [10%] The van der Waals equation of state is, RT 27R?T? RTC p= where and b= - b 2 For Methane Critical Temperature, T. =-82.45C Critical Pressure, Pc = 46.4 bar a a= 64pc 8pc Note: 1 bar = 1.0x105 N/m2 and R= 8.3145 J/mol K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


