Question: 2. (a) Complete the table by utilizing the stoichiometric relationships of the reaction shown below by determining the limiting reactant. Show your work in the
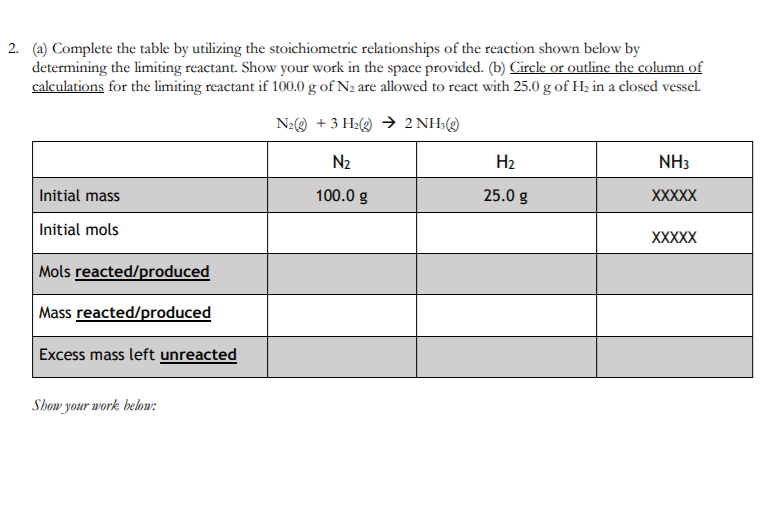
2. (a) Complete the table by utilizing the stoichiometric relationships of the reaction shown below by determining the limiting reactant. Show your work in the space provided. (b) Circle or outline the column of calculations for the limiting reactant if 100.0 g of N2 are allowed to react with 25.0 g of H2 in a closed vessel. Nz( + 3 H2 2NH:( N2 H2 NH3 Initial mass 100.0 g 25.0 g XXXXX Initial mols XXXXX Mols reacted produced Mass reacted/produced Excess mass left unreacted Show your work below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


