Question: 2 A ( g ) + B ( g ) + 2 C ( g ) - > 2 D ( g ) + E
Ag BgCgDg EsFor the reaction in Question # doubling the concentration of A doubles the rate, tripling the concentration of B has no effect, and doubling the concentration of C increases the rate by a factor of By what factor will the rate change if the concentrations of A B and C are all halved?
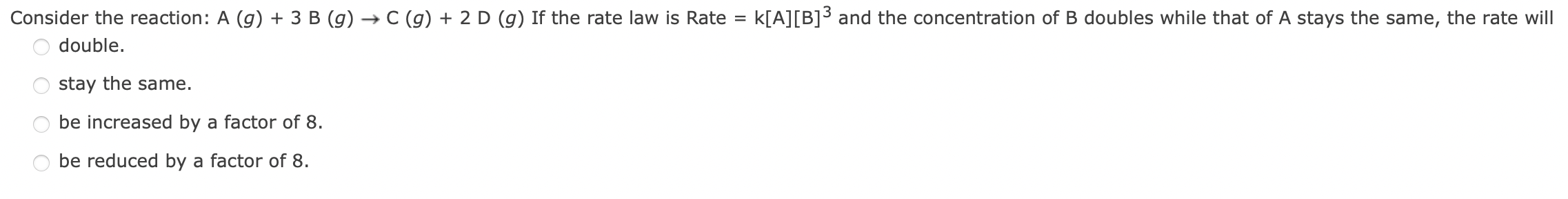
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


