Question: = 2. A monoprotic acid (pKa = 5; total concentration = 0.001 M) is titrated with a strong base. Calculate and draw the titration curve.
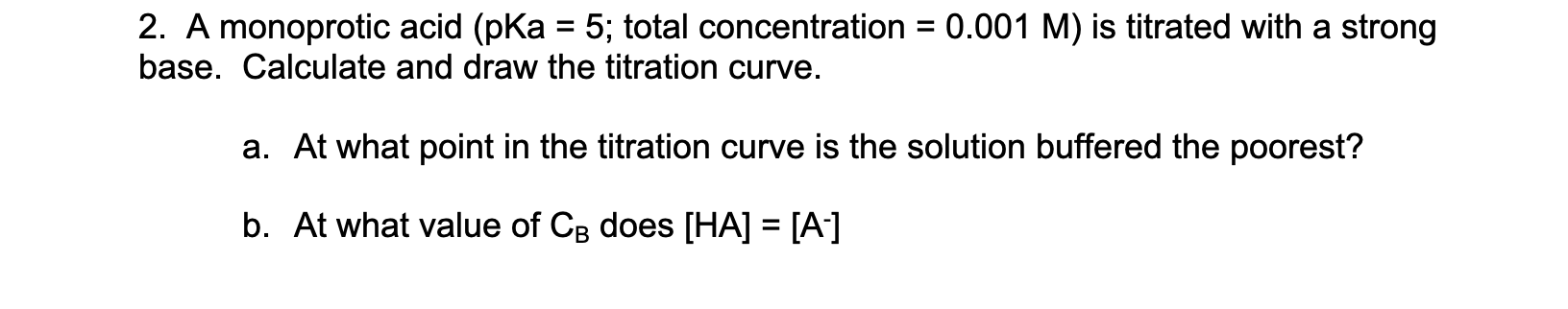
= 2. A monoprotic acid (pKa = 5; total concentration = 0.001 M) is titrated with a strong base. Calculate and draw the titration curve. a. At what point in the titration curve is the solution buffered the poorest? b. At what value of CB does [HA] = [A-] =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


