Question: Determine the required heat input ( in k W ) when 1 0 0 m o l s of toluene is heated from 2 5
Determine the required heat input in when of toluene is heated
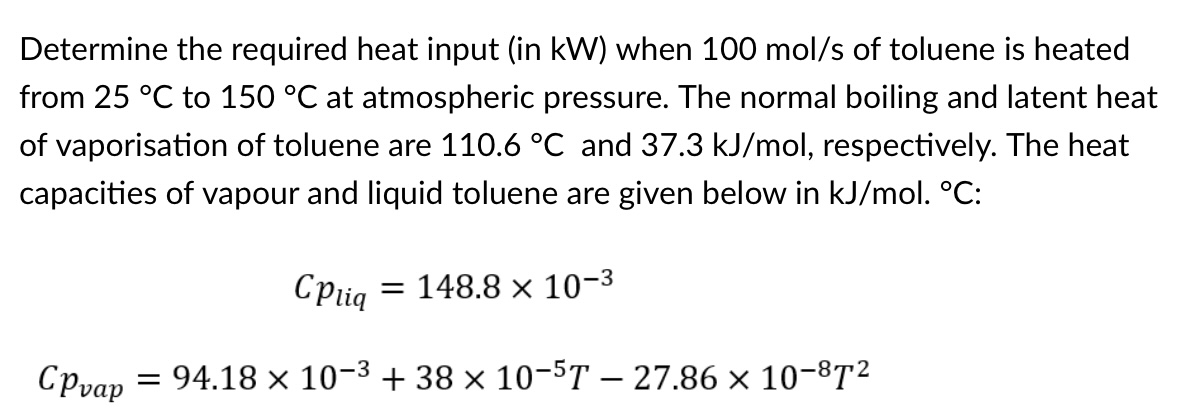
from to at atmospheric pressure. The normal boiling and latent heat
of vaporisation of toluene are and respectively. The heat
capacities of vapour and liquid toluene are given below in :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


