Question: 2. Consider a polystyrene (PS) polymer chain inside a melt of other identical polymers of molecular weight Mw = 106 g/mol and end-to-end size R
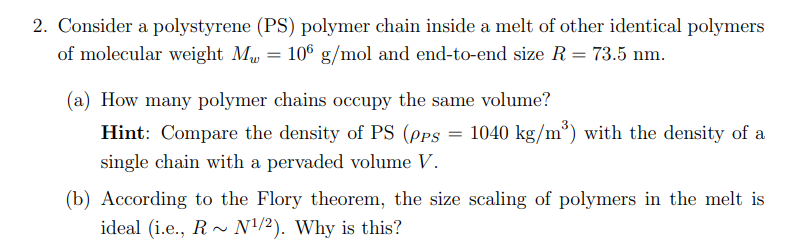
2. Consider a polystyrene (PS) polymer chain inside a melt of other identical polymers of molecular weight Mw = 106 g/mol and end-to-end size R = 73.5 nm. (a) How many polymer chains occupy the same volume? Hint: Compare the density of PS (pps = 1040 kg/m) with the density of a single chain with a pervaded volume V. (b) According to the Flory theorem, the size scaling of polymers in the melt is ideal (i.e., R~N1/2). Why is this? 2. Consider a polystyrene (PS) polymer chain inside a melt of other identical polymers of molecular weight Mw = 106 g/mol and end-to-end size R = 73.5 nm. (a) How many polymer chains occupy the same volume? Hint: Compare the density of PS (pps = 1040 kg/m) with the density of a single chain with a pervaded volume V. (b) According to the Flory theorem, the size scaling of polymers in the melt is ideal (i.e., R~N1/2). Why is this
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


