Question: 2. Consider Compounds C and D begin{tabular}{|l|l|} hline Compound & hline Diagram & & hline Molar Mass & hline end{tabular} a.
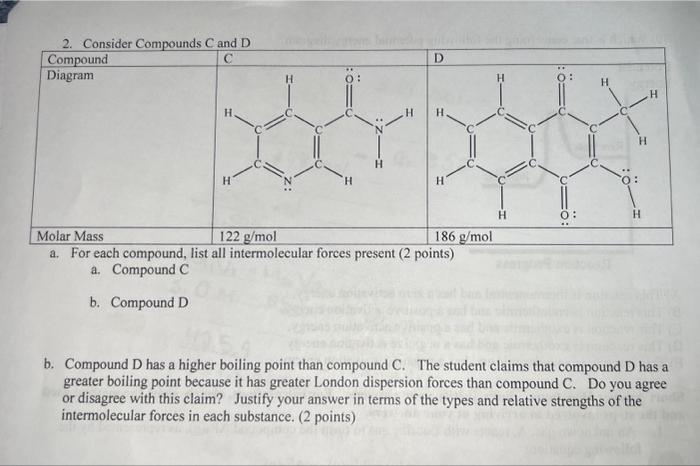
2. Consider Compounds C and D \begin{tabular}{|l|l|} \hline Compound & \\ \hline Diagram & \\ & \\ \hline Molar Mass & \\ \hline \end{tabular} a. For each compound, list all intermolecular forces present ( 2 points) a. Compound C b. Compound D b. Compound D has a higher boiling point than compound C. The student claims that compound D has a greater boiling point because it has greater London dispersion forces than compound C. Do you agree or disagree with this claim? Justify your answer in terms of the types and relative strengths of the intermolecular forces in each substance. (2 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


