Question: 2+ Consider the aqueous phase reaction between the dichromate anion and iron (II) cations: 14 H30+(aq) + Cr20-2-+6Fe2+(aq) - 2Cr3+(aq) + 21H20 What is the
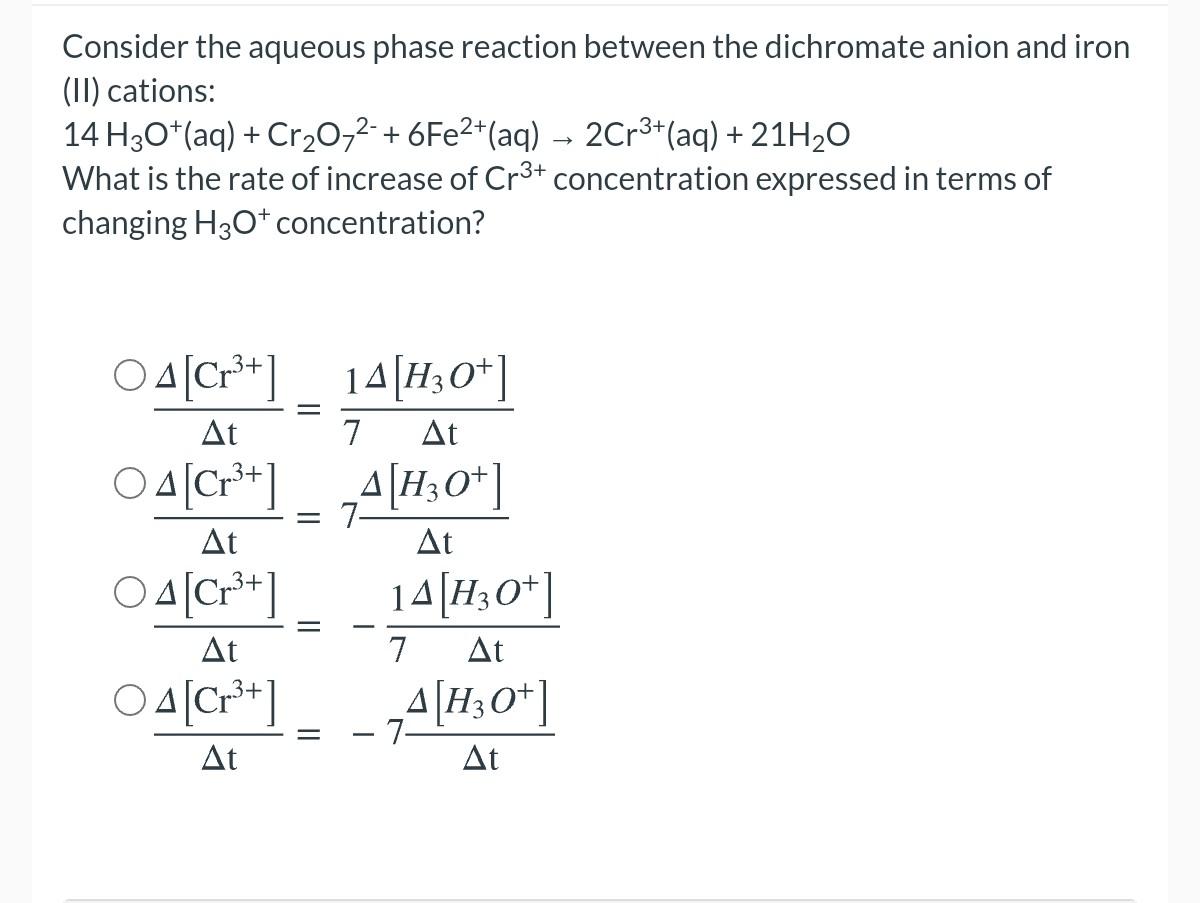
2+ Consider the aqueous phase reaction between the dichromate anion and iron (II) cations: 14 H30+(aq) + Cr20-2-+6Fe2+(aq) - 2Cr3+(aq) + 21H20 What is the rate of increase of Cr3+ concentration expressed in terms of changing H30+ concentration? - O 4 [Cr3+] 14[H3O+] A 7 04[Cr3+1 4[H30+] t At = 7- t t O 4[Cr3+1 14[H30+] = t 7 t O A [Cr3+] A[H3O+] + -7 t t
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


