Question: + = Consider the aqueous phase reaction 72A + B R. The following data has been recorded in a batch reactor for this reaction at
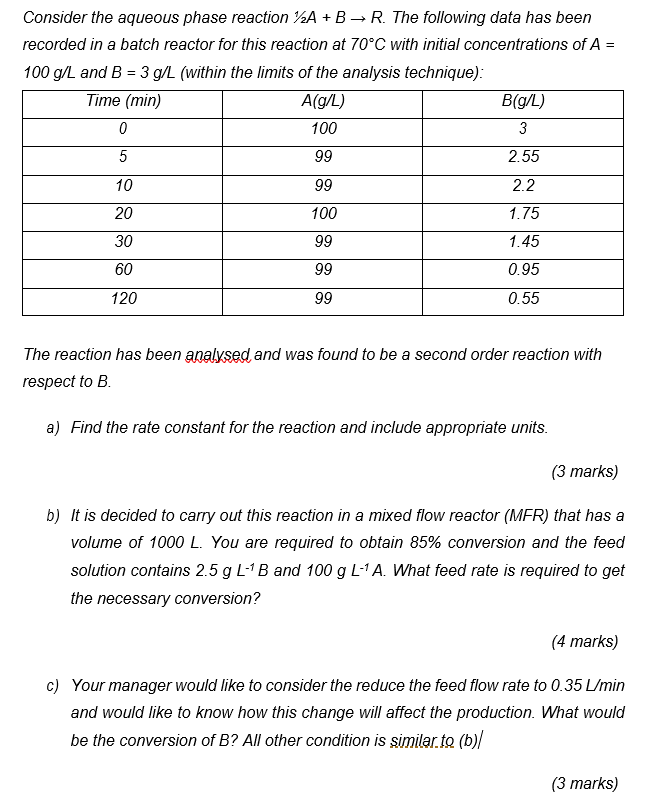
+ = Consider the aqueous phase reaction 72A + B R. The following data has been recorded in a batch reactor for this reaction at 70C with initial concentrations of A = 100 g/L and B = 3 g/L (within the limits of the analysis technique): Time (min) A(g/L) B(g/L) 0 100 3 5 99 2.55 10 99 2.2 20 100 1.75 30 99 1.45 60 99 0.95 120 99 0.55 The reaction has been analysed and was found to be a second order reaction with respect to B. a) Find the rate constant for the reaction and include appropriate units. (3 marks) b) It is decided to carry out this reaction in a mixed flow reactor (MER) that has a volume of 1000 L. You are required to obtain 85% conversion and the feed solution contains 2.5 g L-B and 100 g L-A. What feed rate is required to get the necessary conversion? (4 marks) c) Your manager would like to consider the reduce the feed flow rate to 0.35 L/min and would like to know how this change will affect the production. What would be the conversion of B? All other condition is similar to (b)/
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


