Question: 2) Consider the table below for diffusion of C in Fe and answer the questions that follow: Diffusing Species Host Metal BCC Fe FCC Fe
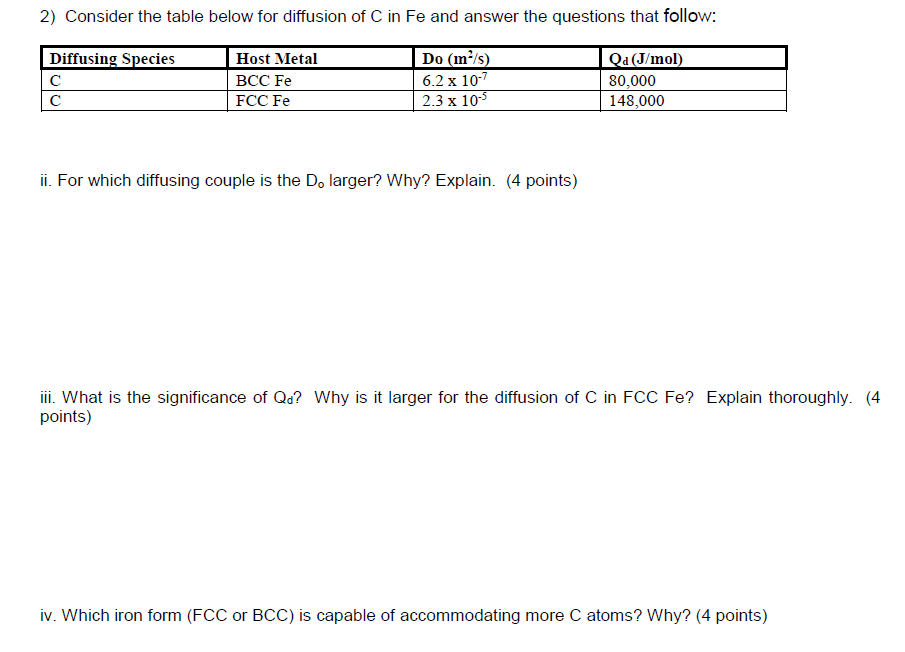
2) Consider the table below for diffusion of C in Fe and answer the questions that follow: Diffusing Species Host Metal BCC Fe FCC Fe Do (ms) 6.2 x 10-7 2.3 x 10 Qa (J/mol) 80,000 148,000 ii. For which diffusing couple is the D, larger? Why? Explain. (4 points) iii. What is the significance of Qd? Why is it larger for the diffusion of C in FCC Fe? Explain thoroughly. (4 points) iv. Which iron form (FCC or BCC) is capable of accommodating more C atoms? Why? (4 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


