Question: 2. For the reaction sequence ki NsOs NO2 + NO3 hy NO. + NO3 NO NO2 +03 NO; + O2 ke 2NO; 2NO: + 02
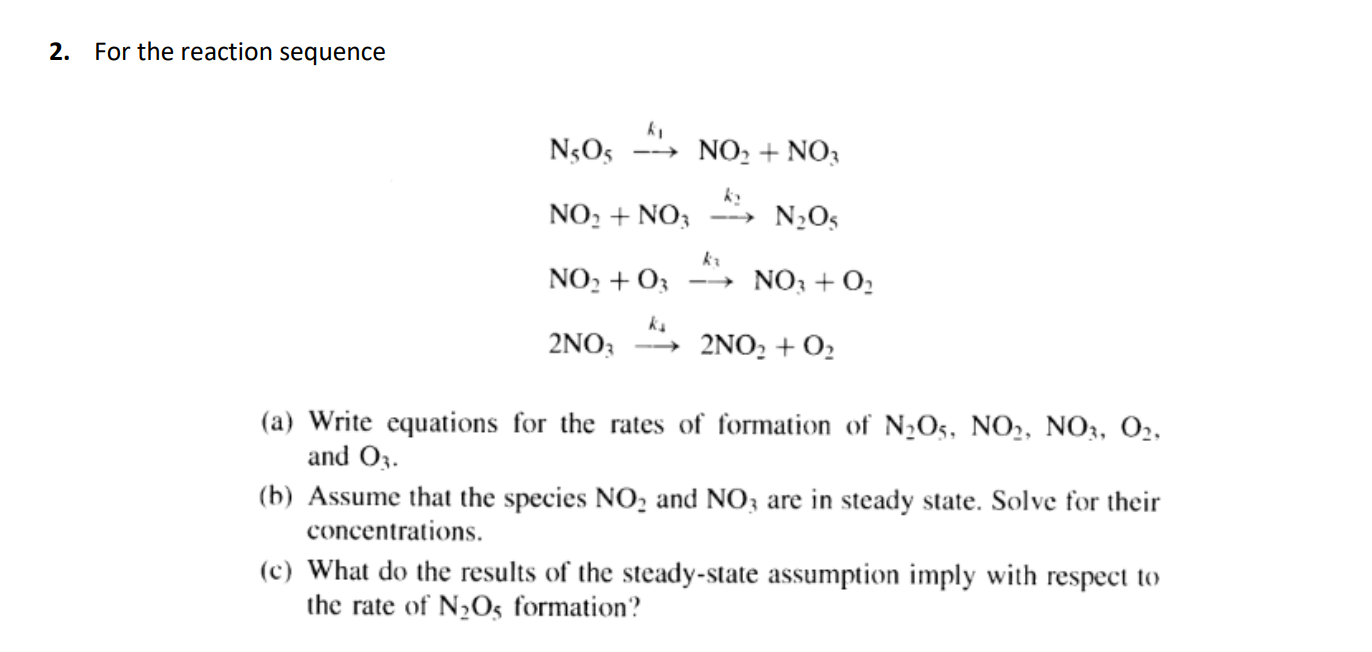
2. For the reaction sequence ki NsOs NO2 + NO3 hy NO. + NO3 NO NO2 +03 NO; + O2 ke 2NO; 2NO: + 02 (a) Write equations for the rates of formation of N2O5, NO, NO3, 02, and 03. (b) Assume that the species NO, and NO3 are in steady state. Solve for their concentrations. (c) What do the results of the steady-state assumption imply with respect to the rate of N2O5 formation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


