Question: 2) Given the following solutions, which solution has the lowest water concentration? Which two have the same osmolarity? 3) Assume that a membrane separating two
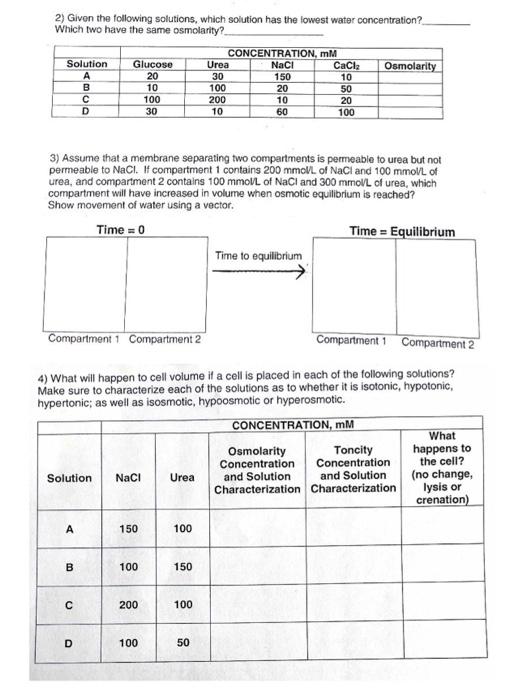
2) Given the following solutions, which solution has the lowest water concentration? Which two have the same osmolarity? 3) Assume that a membrane separating two compertments is permeable to urea but not permeable to NaCl. If compertment 1 contains 200mmolL of NaCl and 100mmolL of urea, and compartment 2 contains 100mmolL of NaCl and 300mmolL of urea, which compartment will have increased in volume when osmotic equilibrium is reached? Show movement of water using a vector. 4) What will happen to cell volume if a cell is placed in each of the following solutions? Make sure to characterize each of the solutions as to whether it is isotonic, hypotonic, hypertonic; as well as isosmotic, hypoosmotic or hyperosmotic
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


