Question: 2 N20 + O2 + 2 N2 + 202 2 CO + O2 2 CO2 automobile catalytic converter A catalytic converter is required on all
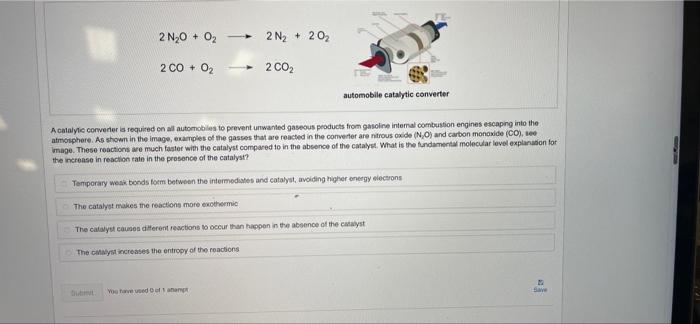
2 N20 + O2 + 2 N2 + 202 2 CO + O2 2 CO2 automobile catalytic converter A catalytic converter is required on all automobiles to prevent unwanted gaseous products from gasoline internal combustion engines escaping into the atmosphere. As shown in the image, examples of the gasses that are reacted in the converter are nitrous oxide (NO) and carbon monoxide (CO), image. These reactions we much faster with the catalyst compared to in the absence of the catalyst. What is the fundamental molecular level explanation for the increase in reaction rate in the presence of the catalyst? Temporary weakbonds form between the intermediates and catalyst, avoiding higher energy electrons The catalyst makes the reactions more exothermic The catalyst causes different reactions to occur than happen in the absence of the catalyst The lyst increases the entropy of the reactions You have done Save
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


