Question: 2) Nitrogen dioxide decomposes according to the equation below. If 1.500 moles of NO2 gas is placed in a container and allowed to reach equilibrium,
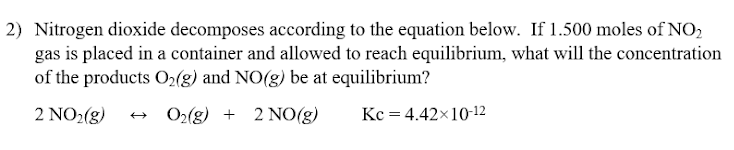
2) Nitrogen dioxide decomposes according to the equation below. If 1.500 moles of NO2 gas is placed in a container and allowed to reach equilibrium, what will the concentration of the products O2(g) and NO(g) be at equilibrium? 2 NO2(g) O2(g) + 2NO(g) Kc = 4.42x10-12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


