Question: 2 NO(g) + O2(g) +2 NO2(9) Reactant Initial Concentration NO 0.0028 M 02 0.0014 M The oxidation of NO(g) producing NO2(g) is represented by the
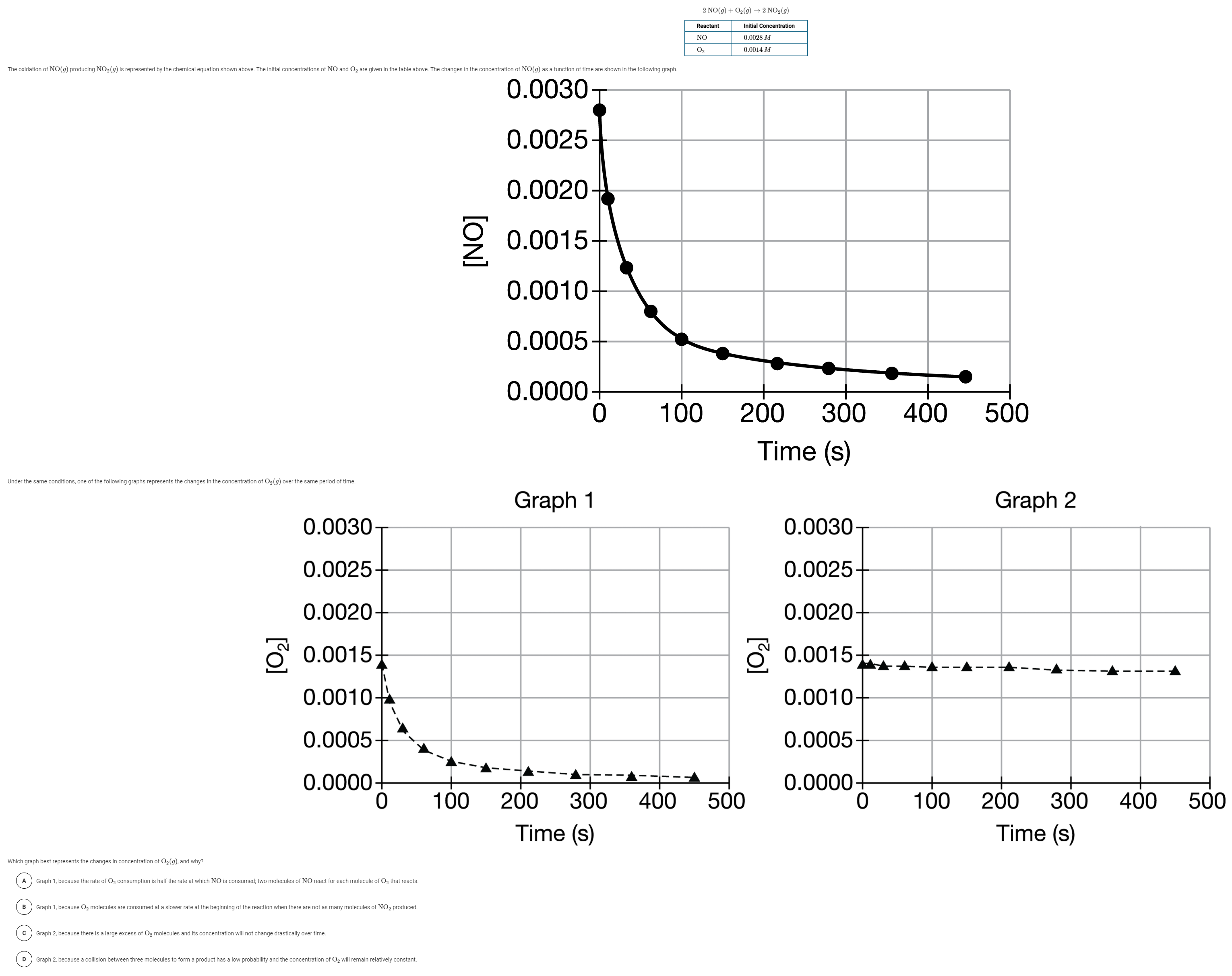
2 NO(g) + O2(g) +2 NO2(9) Reactant Initial Concentration NO 0.0028 M 02 0.0014 M The oxidation of NO(g) producing NO2(g) is represented by the chemical equation shown above. The initial concentrations of NO and O2 are given in the table above. The changes in the concentration of NO(g) as a function of time are shown in the following graph. 0.0030 0.0025 0.0020 2 0.0015 0.0010- 0.0005 0.0000 0 100 200 300 400 500 Time (s) Under the same conditions, one of the following graphs represents the changes in the concentration of O2(9) over the same period of time. Graph 1 Graph 2 0.0030 0.0030 0.0025 0.0025 0.0020 0.0020 0.0015 0.0015 - 0.0010 0.0010 0.0005 0.0005 0.0000 0 0.0000 0 100 200 300 400 500 100 400 500 200 300 Time (s) Time (s) Which graph best represents the changes in concentration of O2(g), and why? A Graph 1, because the rate of Oz consumption is half the rate at which NO is consumed; two molecules of NO react for each molecule of O, that reacts. B Graph 1, because O2 molecules are consumed at a slower rate at the beginning of the reaction when there are not as many molecules of NO2 produced. Graph 2, because there is a large excess of Oz molecules and its concentration will not change drastically over time. D Graph 2, because a collision between three molecules to form a product has a low probability and the concentration of O2 will remain relatively constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


