Question: 2 of 5 Consider the point on the plot where 10.0g of NaOH have been added. What amount of NaOH , in moles, has been
2 of 5\ Consider the point on the plot where
10.0gof
NaOHhave been added. What amount of
NaOH, in moles, has been added?\
0.308molFeCl_(3)initially present\
molNaOH 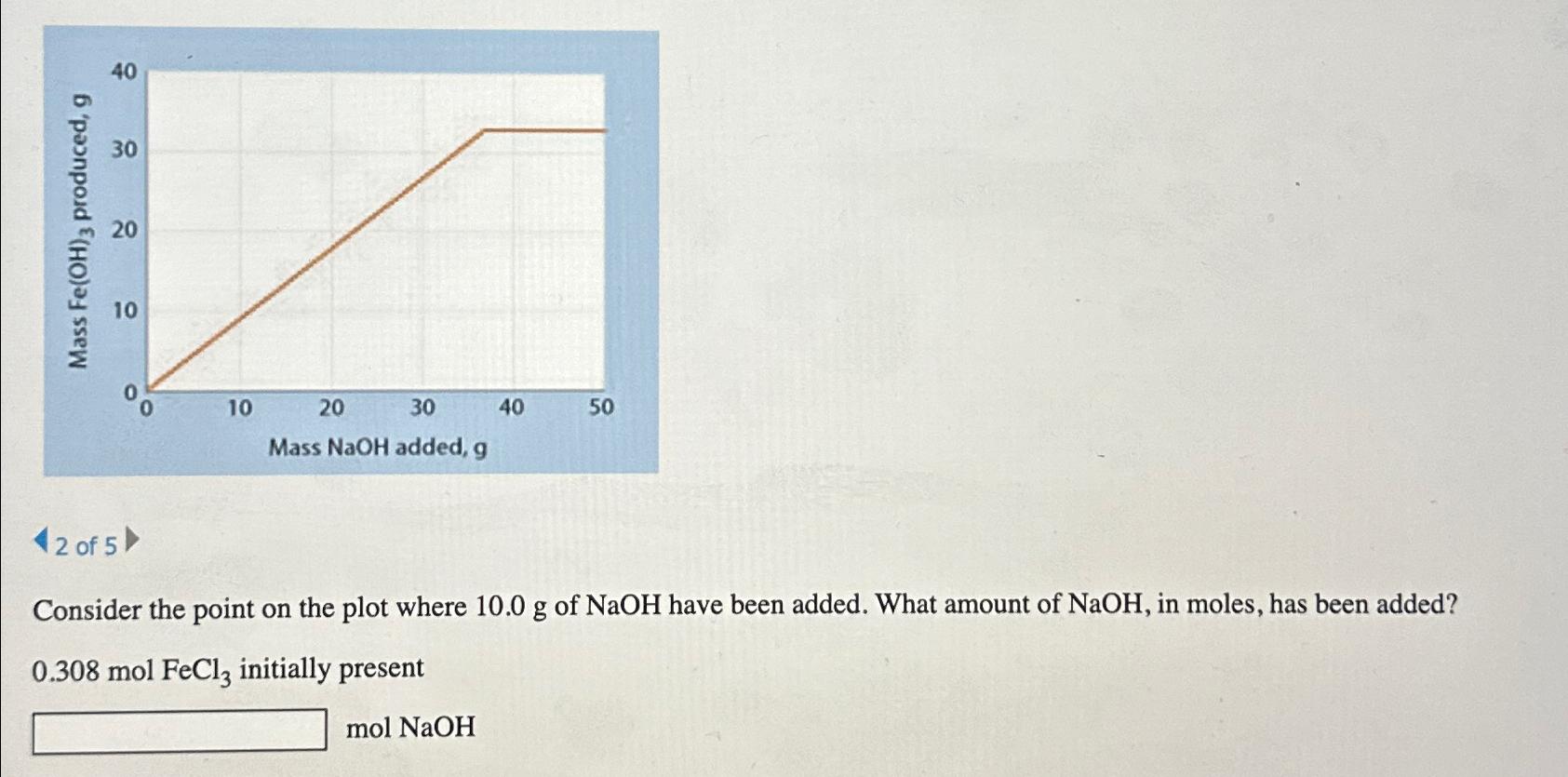
Consider the point on the plot where 10.0g of NaOH have been added. What amount of NaOH, in moles, has been added? 0.308molFeCl3 initially present molNaOH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


