Question: 2 questions second question only need help with part b Enter your answer in the provided box. A piece of dry ice (solid CO2 )
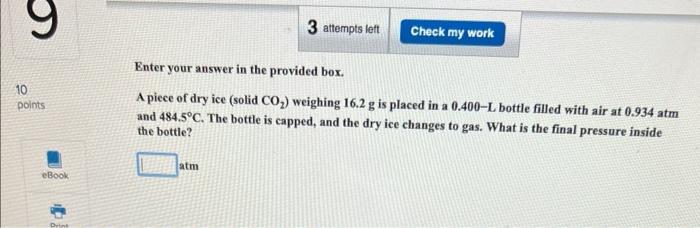
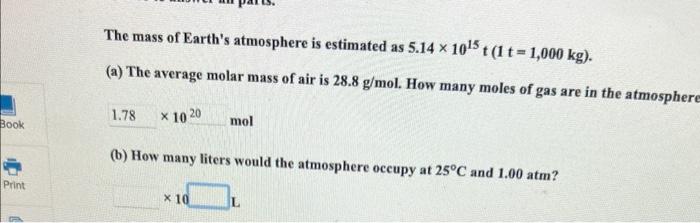
Enter your answer in the provided box. A piece of dry ice (solid CO2 ) weighing 16.2g is placed in a 0.400L bottle filled with air at 0.934atm and 484.5C. The bottle is capped, and the dry ice changes to gas. What is the final pressure inside the bottle? atm The mass of Earth's atmosphere is estimated as 5.141015t(1t=1,000kg). (a) The average molar mass of air is 28.8g/mol. How many moles of gas are in the atmospher 1020mol (b) How many liters would the atmosphere occupy at 25C and 1.00atm ? 10 L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


