Question: 2). The elementary liquid phase reaction 2A+3B2C+D It is carried out isothermally in a batch reactor. a). Draw the appropriate stoichiometric table. b). Calculate the
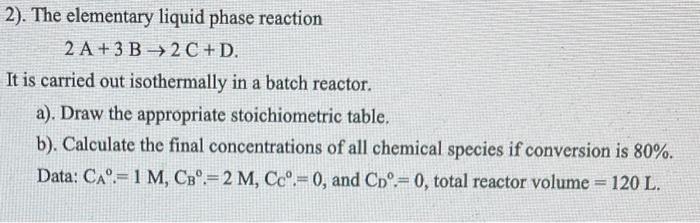
2). The elementary liquid phase reaction 2A+3B2C+D It is carried out isothermally in a batch reactor. a). Draw the appropriate stoichiometric table. b). Calculate the final concentrations of all chemical species if conversion is 80%. Data: CA=1M,CB0=2M,C.=0, and CD.=0, total reactor volume =120L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


