Question: 2. The following data for the reaction BF, + NH, => F,BNH, were measured at 600. K. Experiment [BF), M [NH], M Initial Rate, Ms
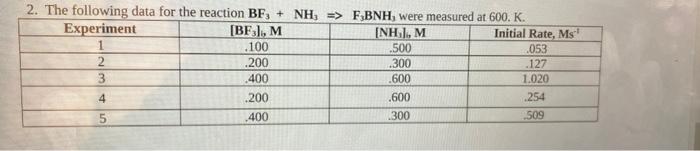
![F,BNH, were measured at 600. K. Experiment [BF), M [NH], M Initial](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9458ddfdb3_30166f9458d92451.jpg)
2. The following data for the reaction BF, + NH, => F,BNH, were measured at 600. K. Experiment [BF), M [NH], M Initial Rate, Ms 1 .100 .500 .053 2 .200 .300 .127 3 .400 .600 1.020 4 .200 .600 .254 5 .400 1300 509 UWN a. Determine the differential rate law for this reaction. b. What is the overall order for this reaction? C. Calculate the value of the rate constant. d. What is the reaction rate when [BF3] = .145 M and (NH) = 400 M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


