Question: 2 The maximum temperature attainable when burning hydrocarbon fuels is limited by the dissociation of CO2 in the product gases. We will consider the reaction
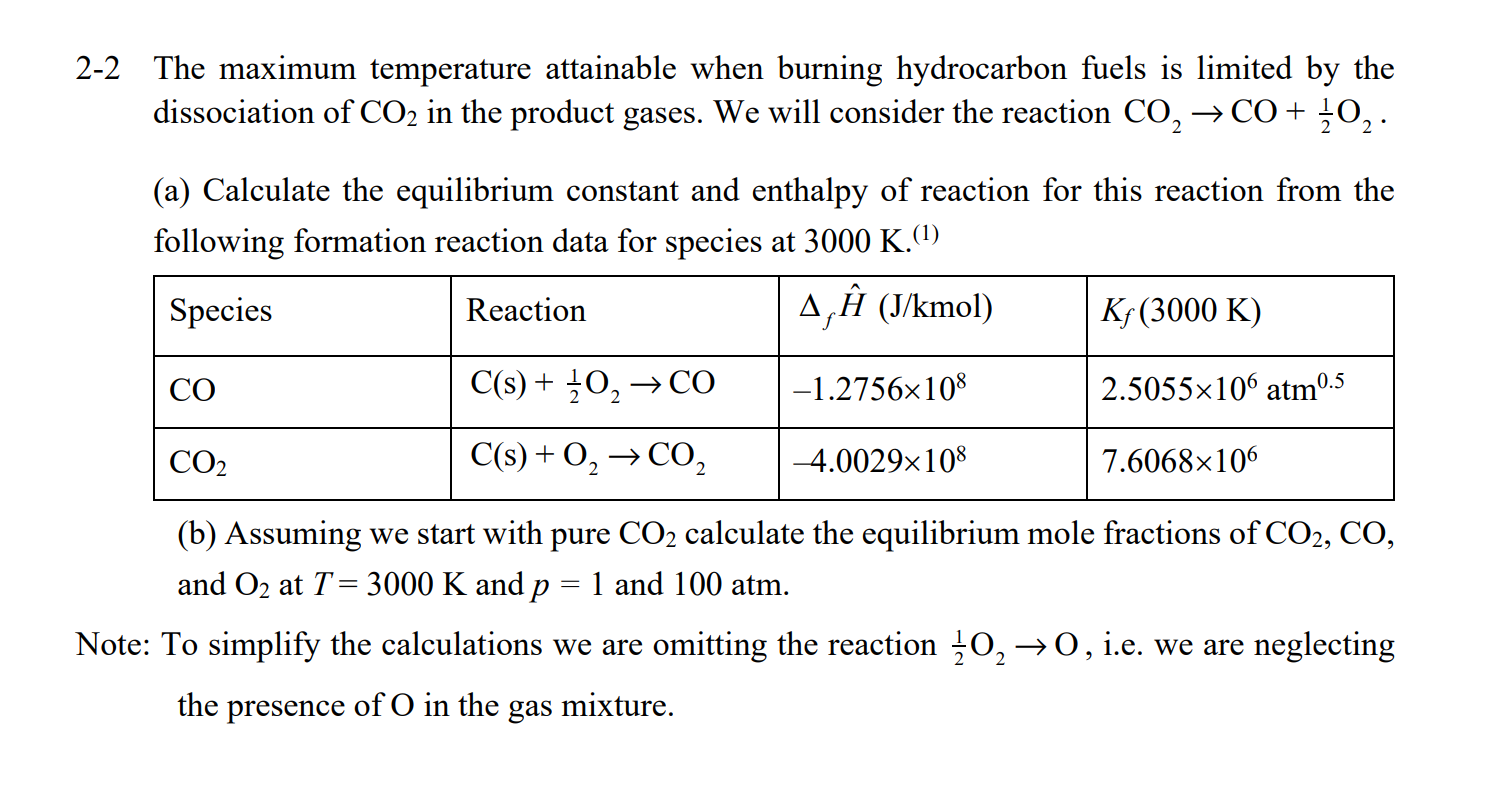
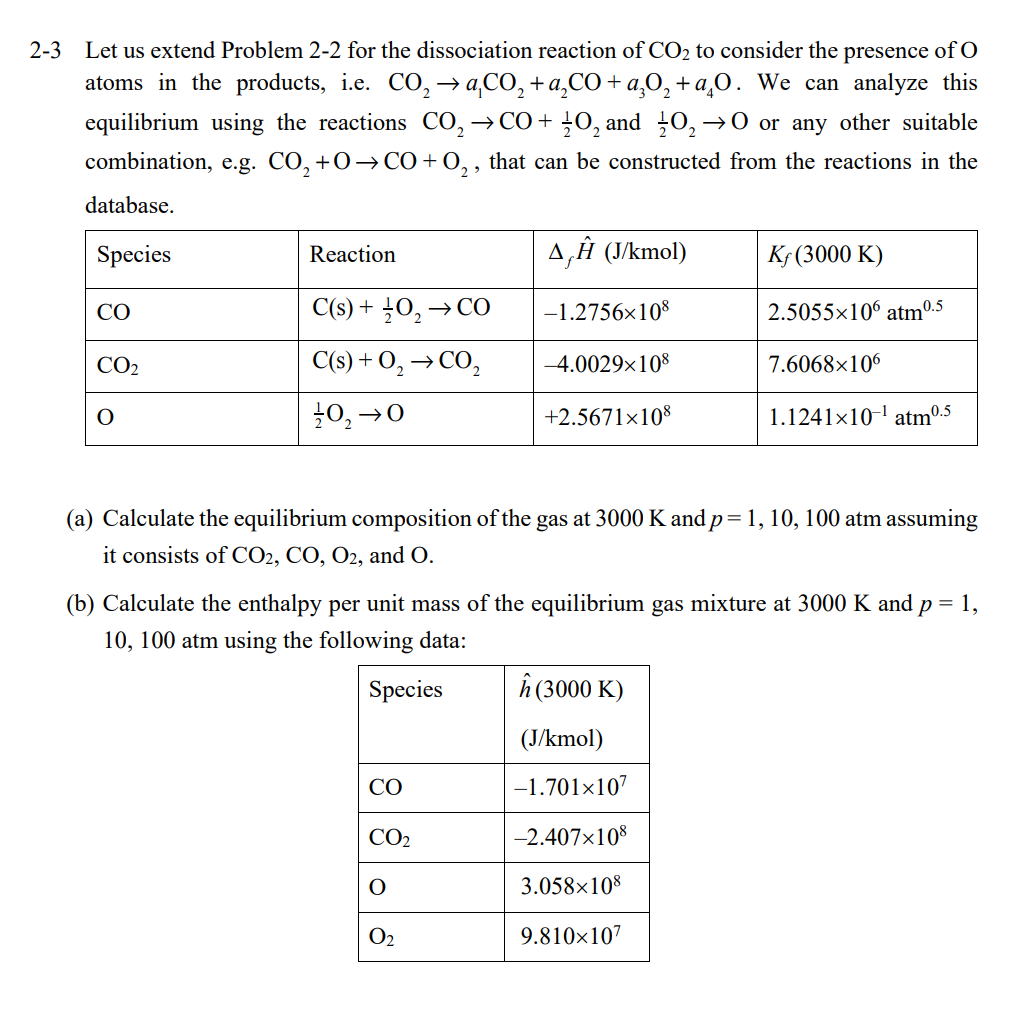
2 The maximum temperature attainable when burning hydrocarbon fuels is limited by the dissociation of CO2 in the product gases. We will consider the reaction CO2CO+21O2. (a) Calculate the equilibrium constant and enthalpy of reaction for this reaction from the following formation reaction data for species at 3000K.(1) (b) Assuming we start with pure CO2 calculate the equilibrium mole fractions of CO2,CO, and O2 at T=3000K and p=1 and 100 atm. ote: To simplify the calculations we are omitting the reaction 21O2O, i.e. we are neglecting the presence of O in the gas mixture. -3 Let us extend Problem 2-2 for the dissociation reaction of CO2 to consider the presence of O atoms in the products, i.e. CO2a1CO2+a2CO+a3O2+a4O. We can analyze this equilibrium using the reactions CO2CO+21O2 and 21O2O or any other suitable combination, e.g. CO2+OCO+O2, that can be constructed from the reactions in the database. (a) Calculate the equilibrium composition of the gas at 3000K and p=1,10,100 atm assuming it consists of CO2,CO,O2, and O. (b) Calculate the enthalpy per unit mass of the equilibrium gas mixture at 3000K and p=1, 10,100atm using the following data
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


