Question: 2) The rate constants for the electron transfer processes that you drew in Part 1 can be written as 1/2 kus = **v2 (rki) exp
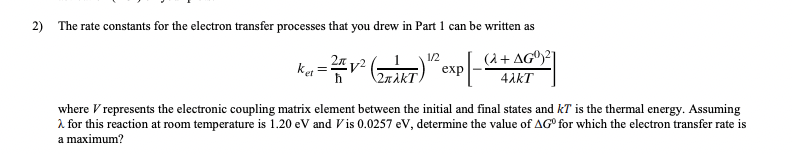
2) The rate constants for the electron transfer processes that you drew in Part 1 can be written as 1/2 kus = **v2 (rki) "exp [-( (+ AG021 4.KT 2KI where V represents the electronic coupling matrix element between the initial and final states and KT is the thermal energy. Assuming 2. for this reaction at room temperature is 1.20 eV and Vis 0.0257 eV, determine the value of AG for which the electron transfer rate is a maximum
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


