Question: Question 3, (10 points) Below, atomic radius, crystal structure, electronegativity, and the most common valence are tabulated, for several metals. Please apply for conditions for
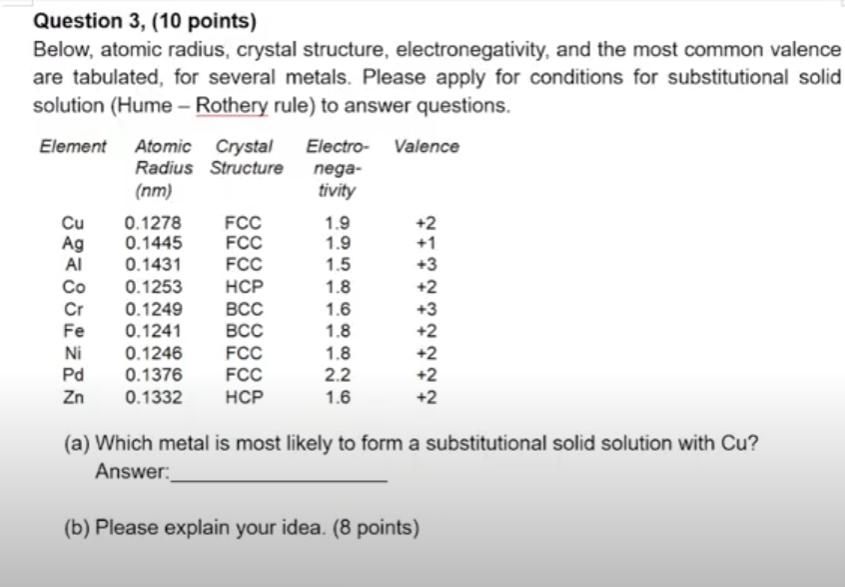
Question 3, (10 points) Below, atomic radius, crystal structure, electronegativity, and the most common valence are tabulated, for several metals. Please apply for conditions for substitutional solid solution (Hume - Rothery rule) to answer questions. Element Atomic Atomic Crystal Electro-Valence Radius Structure nega- (nm) tivity Cu 0.1278 FCC 1.9 +2 Ag 0.1445 FCC 1.9 +1 Al 0.1431 FCC 1.5 +3 Co 0.1253 HCP 1.8 +2 Cr 0.1249 BCC 1.6 +3 Fe 0.1241 BCC 1.8 +2 Ni 0.1246 FCC 1.8 +2 Pd 0.1376 FCC 2.2 +2 Zn 0.1332 HCP 1.6 +2 (a) Which metal is most likely to form a substitutional solid solution with Cu? Answer: (b) Please explain your idea. (8 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


