Question: 22. Circle each central atom that you expect to be sp2-hybridized. H H H Hc C - H-CC=C-H H O: H O: H H H
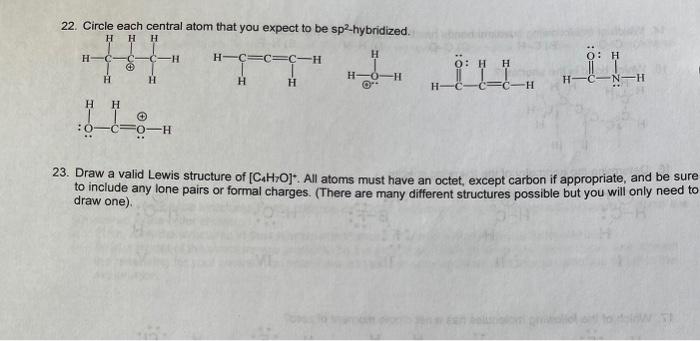
22. Circle each central atom that you expect to be sp2-hybridized. H H H Hc C - H-CC=C-H H O: H O: H H H H-0-H "L H H H H CN-H H -CC H H H 1 :00 0-H 23. Draw a valid Lewis structure of [CHOj. All atoms must have an octet, except carbon if appropriate, and be sure to include any lone pairs or formal charges. (There are many different structures possible but you will only need to draw one)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


