Question: 24. Draw the other resonance structure (maintaining the same overall charge). Show the movement of electrons with curved arrows. Circle the MAJOR resonance form. 25.
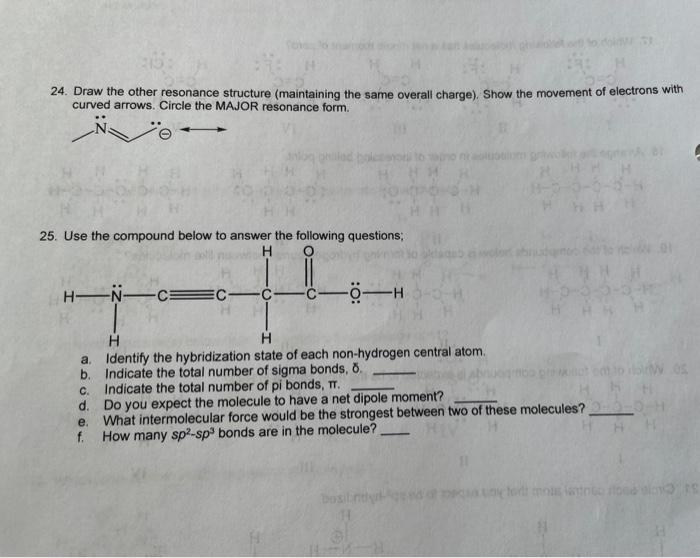
24. Draw the other resonance structure (maintaining the same overall charge). Show the movement of electrons with curved arrows. Circle the MAJOR resonance form. 25. Use the compound below to answer the following questions, H H-N-C=C -C - H H a. Identify the hybridization state of each non-hydrogen central atom b. Indicate the total number of sigma bonds, 8. c. Indicate the total number of pi bonds, TT, d. Do you expect the molecule to have a net dipole moment? e. What intermolecular force would be the strongest between two of these molecules? 230 f. How many sp-spbonds are in the molecule? Dos
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


