Question: 24. Consider the phase diagram shown. Choose the statement below that is TRUE. a. b. C. d. Pressure (not to scale) 72.9 atm 5.1
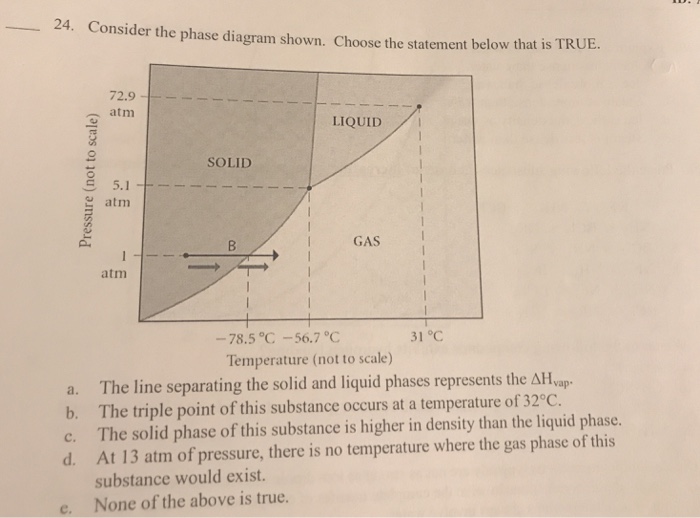
24. Consider the phase diagram shown. Choose the statement below that is TRUE. a. b. C. d. Pressure (not to scale) 72.9 atm 5.1 atm atm SOLID LIQUID GAS 31 C -78.5 C -56.7 C Temperature (not to scale) The line separating the solid and liquid phases represents the AH vap- The triple point of this substance occurs at a temperature of 32C. The solid phase of this substance is higher in density than the liquid phase. At 13 atm of pressure, there is no temperature where the gas phase of this substance would exist. None of the above is true.
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
The correct answer is Letter C The solid phase of t... View full answer

Get step-by-step solutions from verified subject matter experts


