Question: Consider the phase diagram below. Which ONE statement is false? A . The normal melting point is leas tgan 3 7 5 k B .
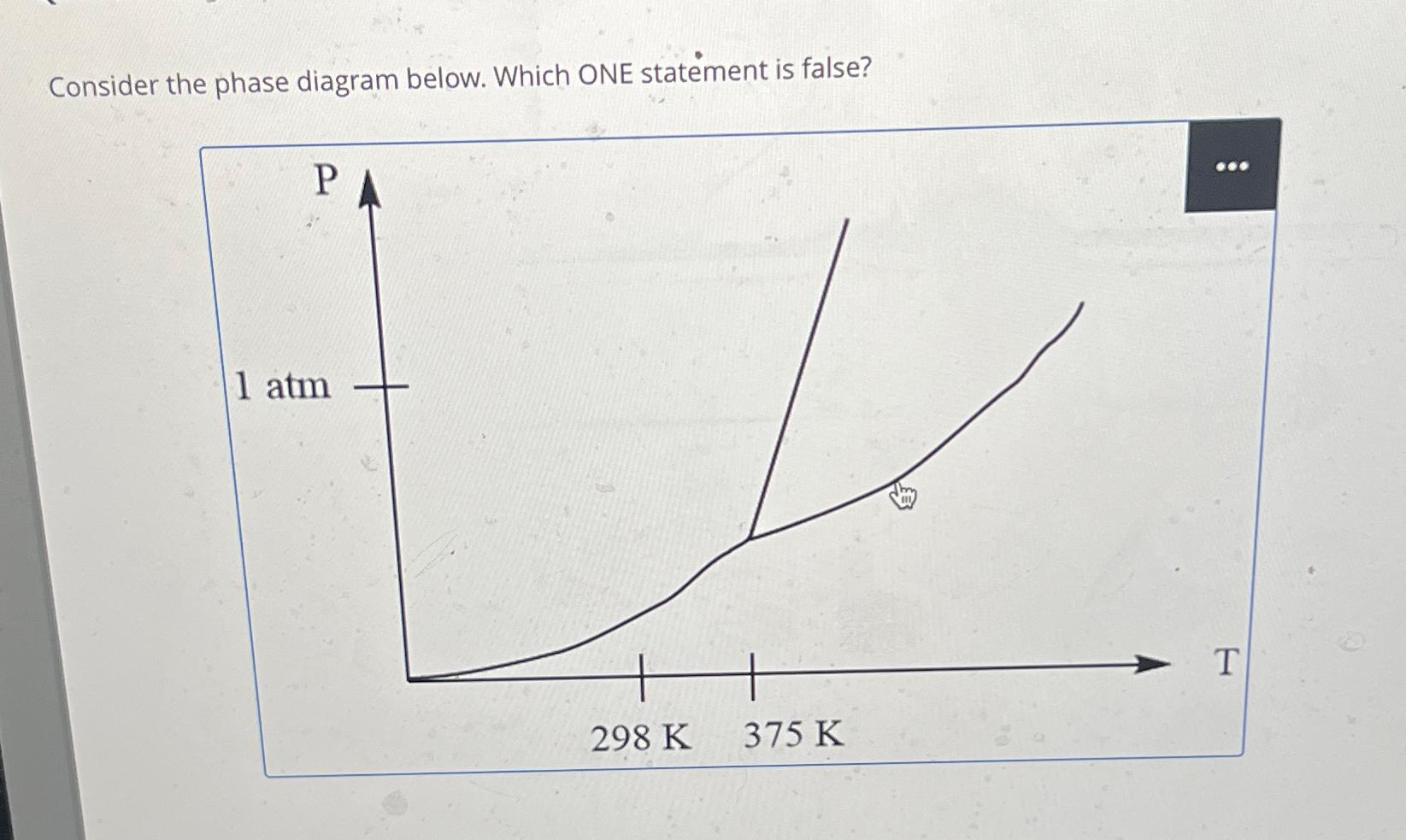
Consider the phase diagram below. Which ONE statement is false?
A The normal melting point is leas tgan k
B The solid phase has a higher density than the liquid phase
C The liqud phase cannot exisit below k
D If a sample of this substance was on a desk in front of you would it be solid
E At atm and k the sunstance is a gas.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


