Question: pleaae answer 10 for me a-f 10. Consider the phase diagram shown below. Temperature (not to scale) a. Which section represents the solid phase? b.
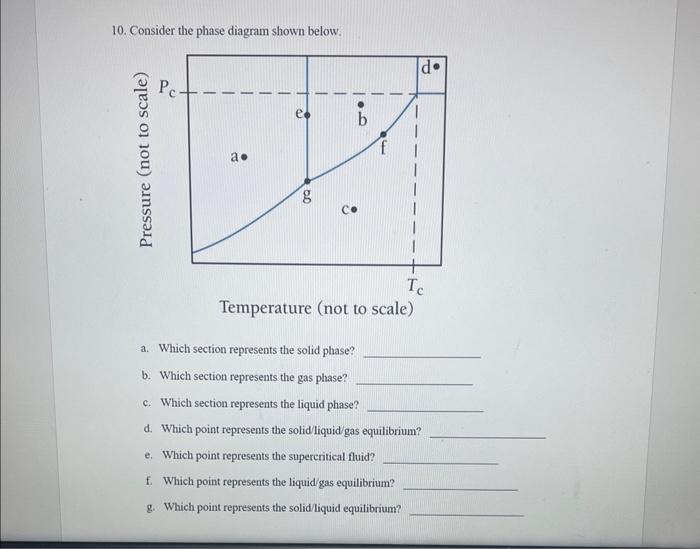
10. Consider the phase diagram shown below. Temperature (not to scale) a. Which section represents the solid phase? b. Which section represents the gas phase? c. Which section represents the liquid phase? d. Which point represents the solid/liquid/gas equilibrium? e. Which point represents the supercritical fluid? f. Which point represents the liquid/gas equilibrium? g. Which point represents the solid/liquid equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


