Question: 25. The point where the hydroxide ion concentration is equal to the hydronium ion concentration for the titration represented by the graph below is called
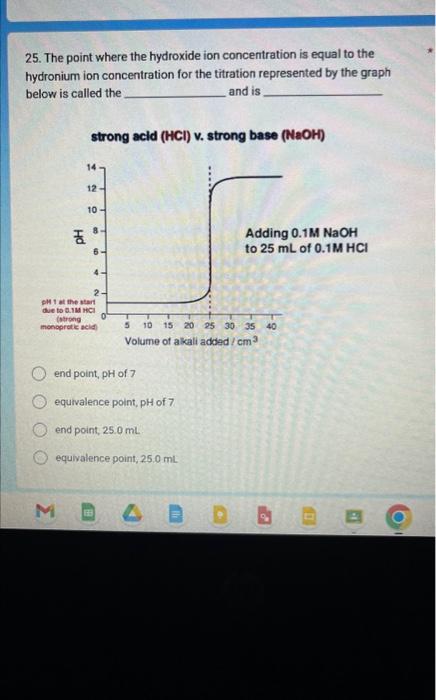
25. The point where the hydroxide ion concentration is equal to the hydronium ion concentration for the titration represented by the graph below is called the and is strong acid (HCl) v. strong base (NaOH) end point, pH of 7 equivalence point, pH of 7 end point, 25.0mL equivalence point, 25.0mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


