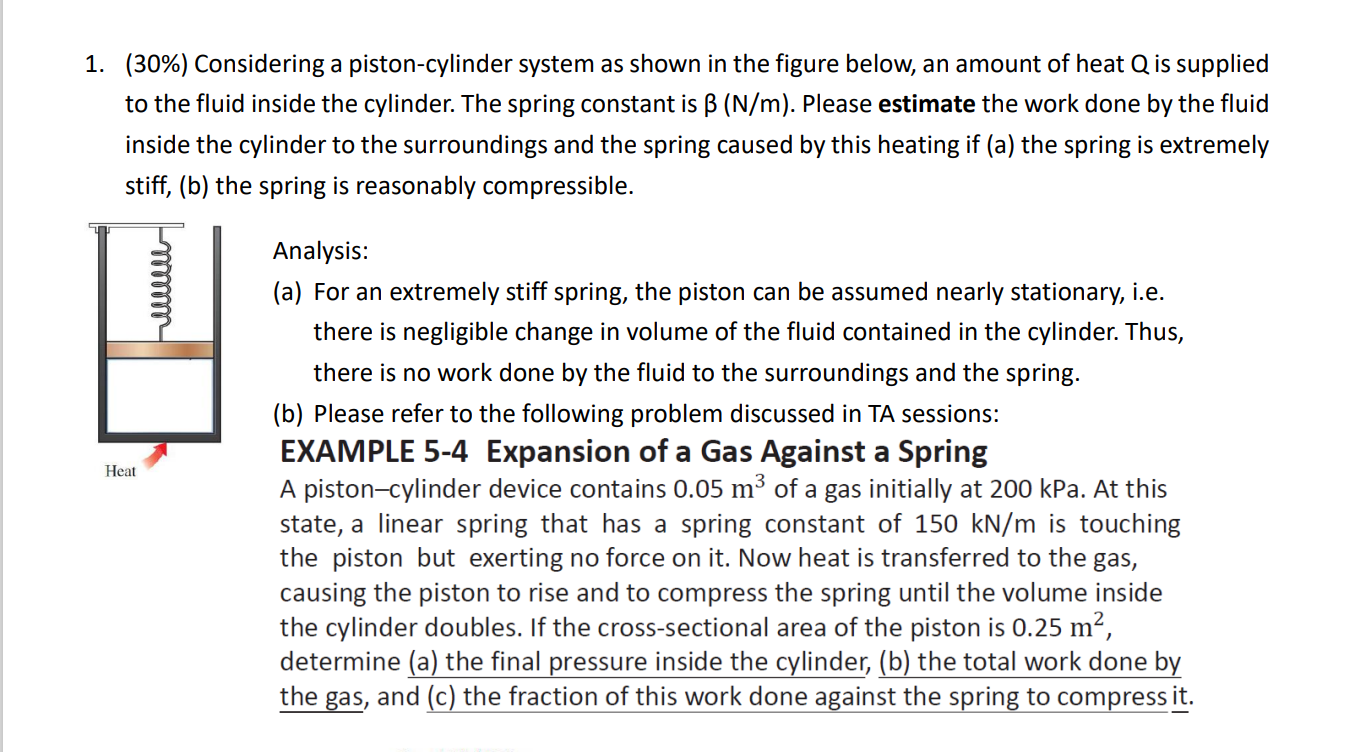
Question: ( 3 0 % ) Considering a piston - cylinder system as shown in the figure below, an amount of heat Q is supplied to
Considering a pistoncylinder system as shown in the figure below, an amount of heat is supplied
to the fluid inside the cylinder. The spring constant is Please estimate the work done by the fluid
inside the cylinder to the surroundings and the spring caused by this heating if a the spring is extremely
stiff, b the spring is reasonably compressible.
Analysis:
a For an extremely stiff spring, the piston can be assumed nearly stationary, ie
there is negligible change in volume of the fluid contained in the cylinder. Thus,
there is no work done by the fluid to the surroundings and the spring.
b Please refer to the following problem discussed in TA sessions:
EXAMPLE Expansion of a Gas Against a Spring
A pistoncylinder device contains of a gas initially at kPa At this
state, a linear spring that has a spring constant of is touching
the piston but exerting no force on it Now heat is transferred to the gas,
causing the piston to rise and to compress the spring until the volume inside
the cylinder doubles. If the crosssectional area of the piston is
determine a the final pressure inside the cylinder, b the total work done by
the gas, and c the fraction of this work done against the spring to compress it
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


