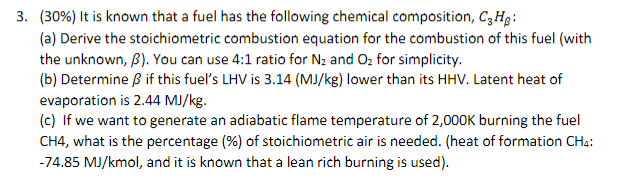
Question: ( 3 0 % ) It is known that a fuel has the following chemical composition, C 3 H : ( a ) Derive the
It is known that a fuel has the following chemical composition, :
a Derive the stoichiometric combustion equation for the combustion of this fuel with
the unknown, You can use : ratio for and for simplicity.
b Determine if this fuel's LHV is lower than its HHV Latent heat of
evaporation is
c If we want to generate an adiabatic flame temperature of burning the fuel
what is the percentage of stoichiometric air is needed. heat of formation :
mol, and it is known that a lean rich burning is used
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


