Question: ( 3 0 pts ) Biodiesel is formed by the reaction of lipids ( fats and oils ) with an alcohol in the oresence of
pts Biodiesel is formed by the reaction of lipids fats and oils with an alcohol in the
oresence of a catalyst usually base However, water is often in the oils and needs to be
emoved because the presence of water will cause the production of soap instead of
jiodiesel. A flash drum is used to remove water from Stearic Acid. A mixture
of mol Stearic Acid SA and mol Water W is flashed isothermally from
oar to bar at Assume Raoult's Law applies an ideal mixture Antione's
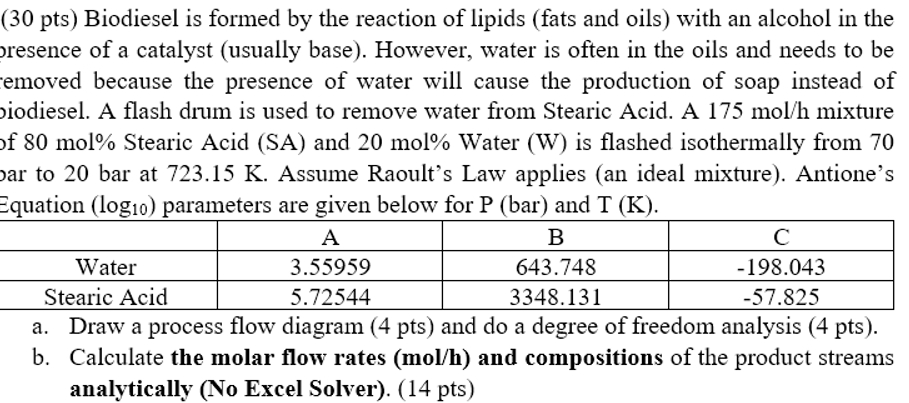
Equation parameters are given below for and
a Draw a process flow diagram pts and do a degree of freedom analysis
b Calculate the molar flow rates and compositions of the product streams
analytically No Excel Solver pts
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


