Question: (30 pts) Biodiesel is formed by the reaction of lipids (fats and oils) with an alcohol in the presence of a catalyst (usually base). However,
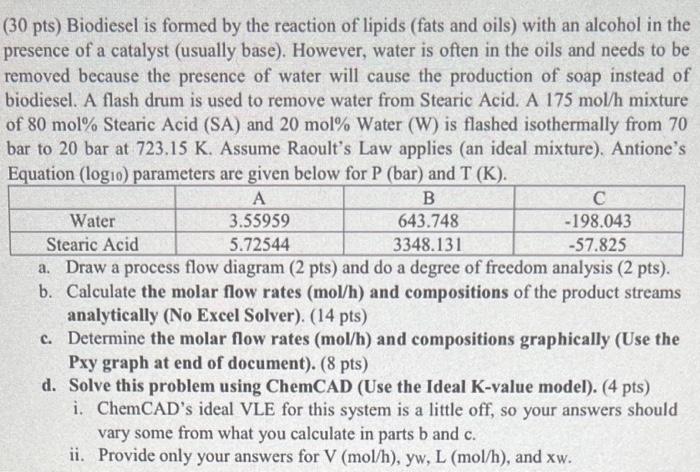
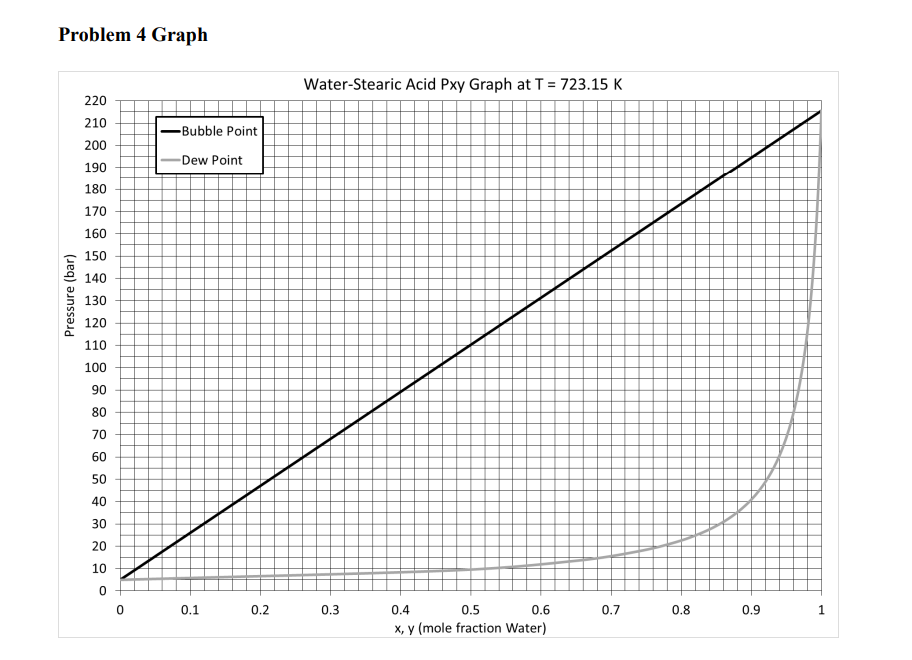
(30 pts) Biodiesel is formed by the reaction of lipids (fats and oils) with an alcohol in the presence of a catalyst (usually base). However, water is often in the oils and needs to be removed because the presence of water will cause the production of soap instead of biodiesel. A flash drum is used to remove water from Stearic Acid. A 175mol/h mixture of 80mol% Stearic Acid (SA) and 20mol% Water (W) is flashed isothermally from 70 bar to 20 bar at 723.15K. Assume Raoult's Law applies (an ideal mixture). Antione's Equation (log10) parameters are given below for P(bar) and T(K). a. Draw a process flow diagram ( 2 pts) and do a degree of freedom analysis ( 2 pts). b. Calculate the molar flow rates (mol/h) and compositions of the product streams analytically (No Excel Solver). (14 pts) c. Determine the molar flow rates (mol/h) and compositions graphically (Use the Pxy graph at end of document). ( 8pts) d. Solve this problem using ChemCAD (Use the Ideal K-value model). (4 pts) i. ChemCAD's ideal VLE for this system is a little off, so your answers should vary some from what you calculate in parts b and c. ii. Provide only your answers for V(mol/h),yw,L(mol/h), and xw. Problem 4 Graph
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


