Question: 3. ( 2 marks) Identify the percent yield when 28.16g of CO2 are formed from the reaction of 4.000 moles of C8H18 with 4.000 moles
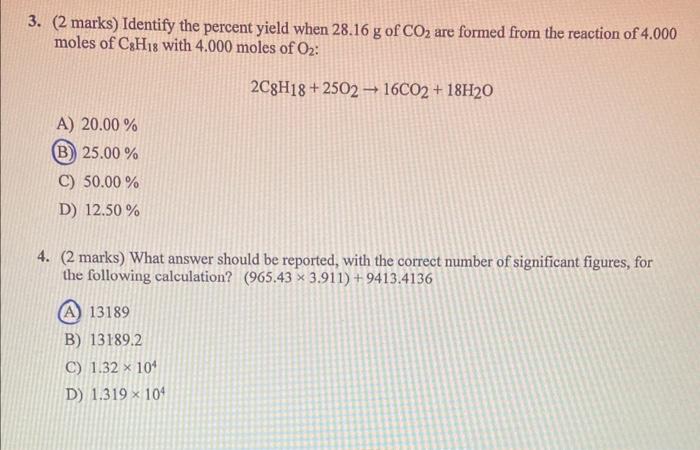
3. ( 2 marks) Identify the percent yield when 28.16g of CO2 are formed from the reaction of 4.000 moles of C8H18 with 4.000 moles of O2 : 2C8H18+25O216CO2+18H2O A) 20.00% (B) 25.00% C) 50.00% D) 12.50% 4. ( 2 marks) What answer should be reported, with the correct number of significant figures, for the following calculation? (965.433.911)+9413.4136 (A) 13189 B) 13189.2 C) 1.32104 D) 1.319104
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


