Question: 3). A compound decomposes with pseudo 1 order kinetics in buffered aqueous solution. The pseudo first order rate constant varies with the pH of the
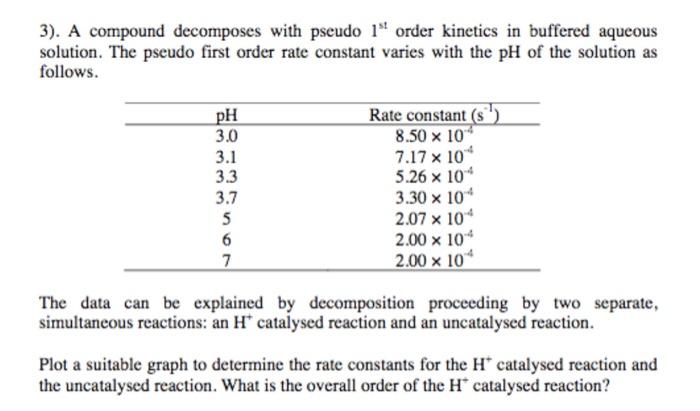
3). A compound decomposes with pseudo 1" order kinetics in buffered aqueous solution. The pseudo first order rate constant varies with the pH of the solution as follows. pH 3.0 3.1 3.3 3.7 5 6 7 Rate constant (s 8.50 x 10 7.17 x 10 5.26 x 10 3.30 x 10 2.07 x 10 2.00 x 10" 2.00 x 10 The data can be explained by decomposition proceeding by two separate, simultaneous reactions: an H* catalysed reaction and an uncatalysed reaction. Plot a suitable graph to determine the rate constants for the H* catalysed reaction and the uncatalysed reaction. What is the overall order of the H* catalysed reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


