Question: 3. (a) Consider the following first order decomposition reactions. Write down the following rates; rA= rR= rS= rT= rU= (b) B is desired product for
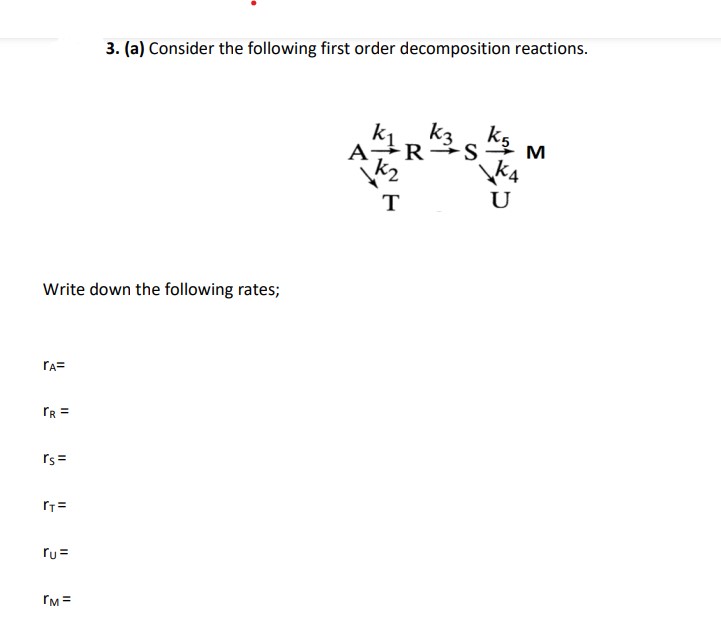
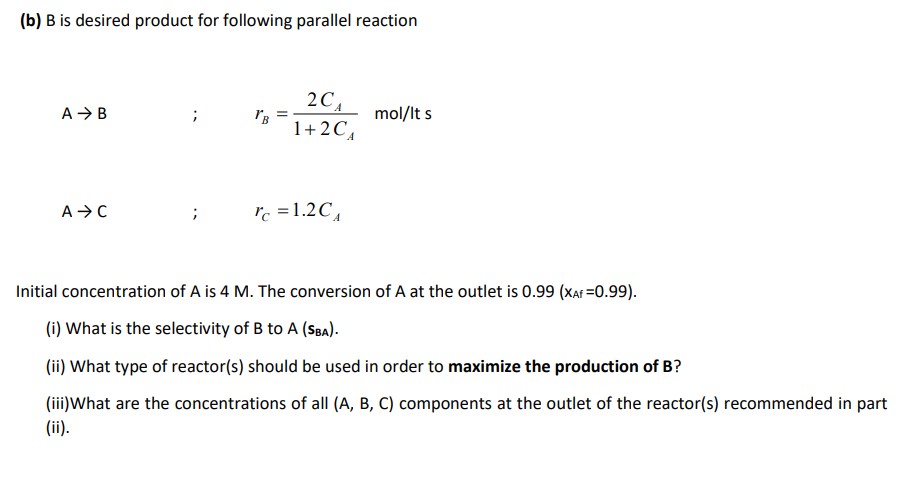
3. (a) Consider the following first order decomposition reactions. Write down the following rates; rA= rR= rS= rT= rU= (b) B is desired product for following parallel reaction ABAC;;rB=1+2CA2CAmol/ltsrC=1.2CA Initial concentration of A is 4M. The conversion of A at the outlet is 0.99(XAf=0.99). (i) What is the selectivity of B to A(sBA). (ii) What type of reactor(s) should be used in order to maximize the production of B ? (iii)What are the concentrations of all (A, B, C) components at the outlet of the reactor(s) recommended in part (ii). 3. (a) Consider the following first order decomposition reactions. Write down the following rates; rA= rR= rS= rT= rU= (b) B is desired product for following parallel reaction ABAC;;rB=1+2CA2CAmol/ltsrC=1.2CA Initial concentration of A is 4M. The conversion of A at the outlet is 0.99(XAf=0.99). (i) What is the selectivity of B to A(sBA). (ii) What type of reactor(s) should be used in order to maximize the production of B ? (iii)What are the concentrations of all (A, B, C) components at the outlet of the reactor(s) recommended in part (ii)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


