Question: (a) Consider the following first order decomposition reactions. Write down the following rates; T= 15= 1+2C k (b) B is desired product for following parallel
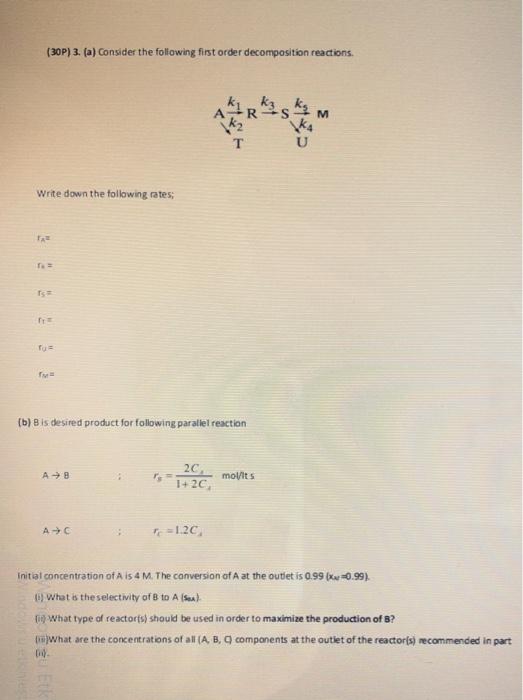
(30P) 3. (a) Consider the following first order decomposition reactions. TAk2k2k1Rk3USk4k4k5M Write down the following rates; rn=rn=r5=r1=r0=rm= (b) B is desired product for following parallel reaction A8;r5=1+2C42C2molftsAC;TC=1.2CA initial concentration of A is 4M. The canversion of A at the outlet is 0.99(xw=0.99). (i) What is the selectivity of B to A (sea). (ii) What type of reactoris) should be used in order to maximize the production of B ? (ini)What are the concentrations of all (A, B, Q companents at the outiet of the reactor(s) necommended in part (ii)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


