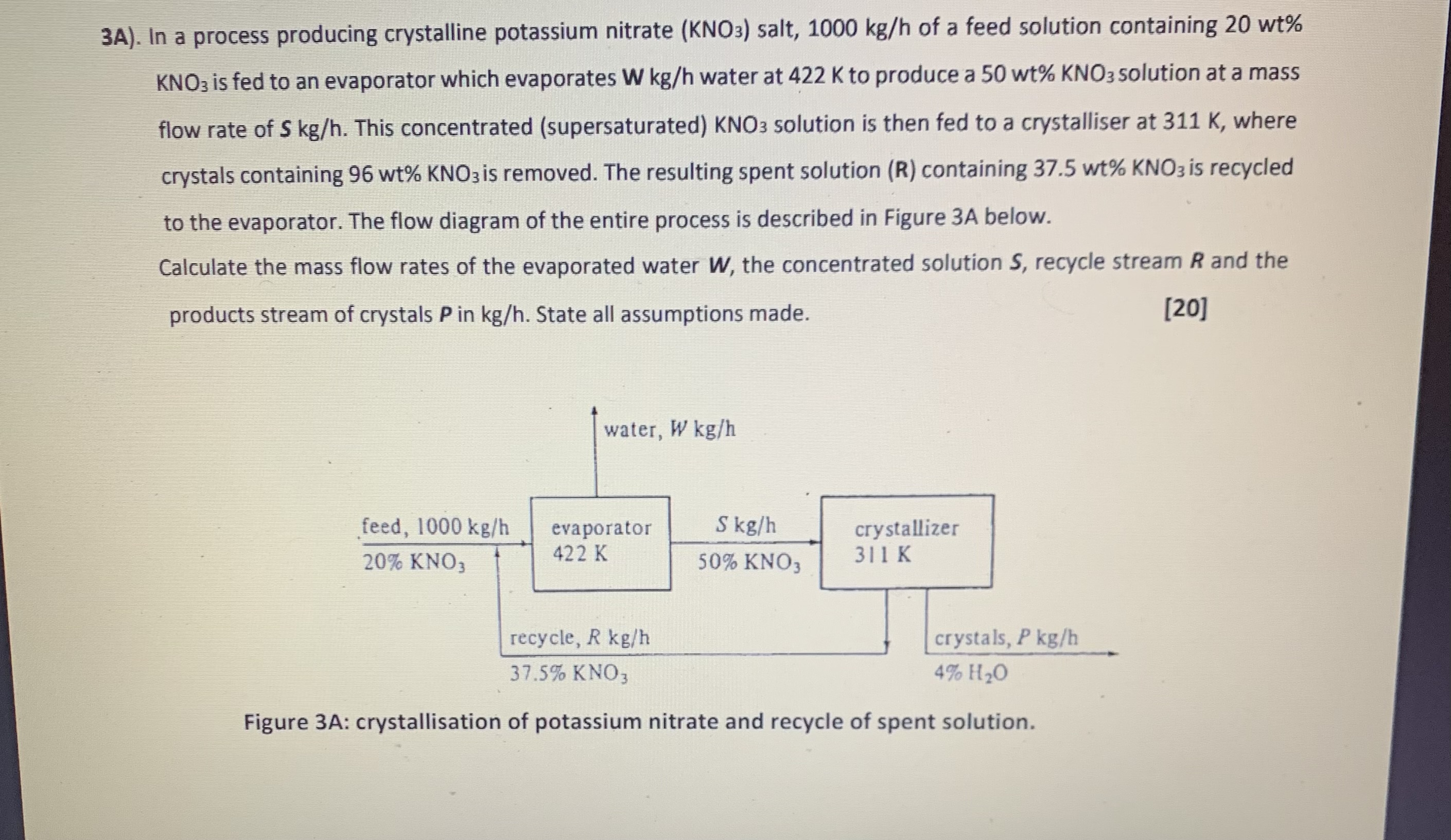
Question: 3 A ) . In a process producing crystalline potassium nitrate ( K N O 3 ) salt, 1 0 0 0 k g h
A In a process producing crystalline potassium nitrate salt, of a feed solution containing
is fed to an evaporator which evaporates water at to produce a solution at a mass
flow rate of This concentrated supersaturated solution is then fed to a crystalliser at where
crystals containing is removed. The resulting spent solution containing is recycled
to the evaporator. The flow diagram of the entire process is described in Figure A below.
Calculate the mass flow rates of the evaporated water the concentrated solution recycle stream and the
products stream of crystals in State all assumptions made.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


