Question: Instructions: 1. Solve these problems using Microsoft Excel. 2. The final excel files must be uploaded to the blackboard (assignment section) before the deadline. 3.

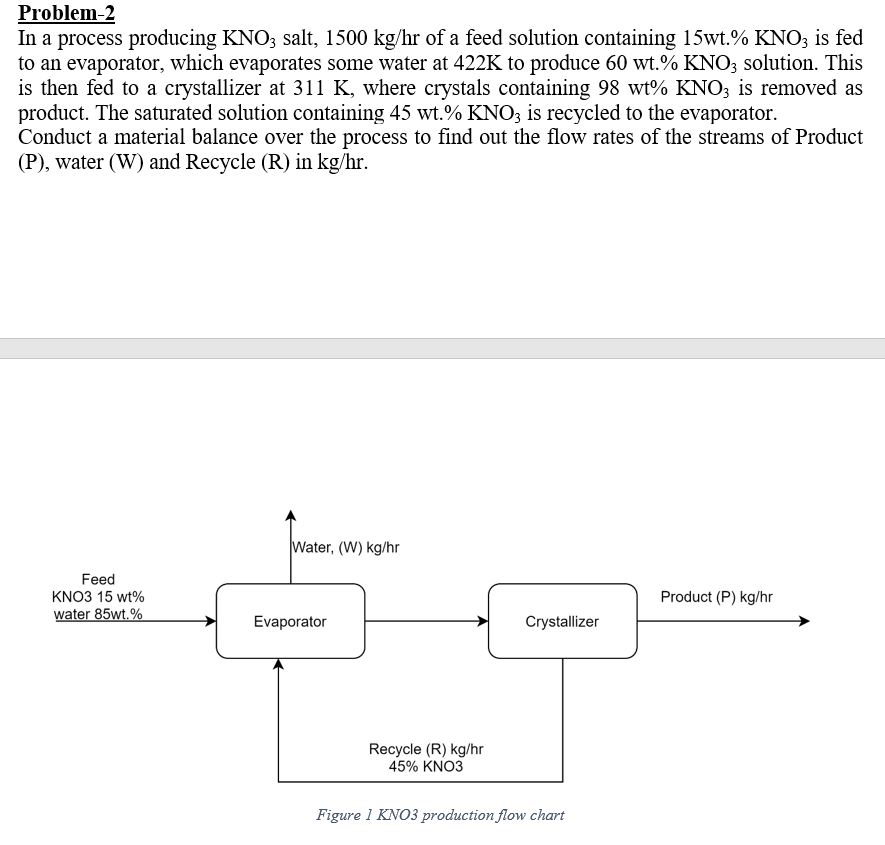
Instructions: 1. Solve these problems using Microsoft Excel. 2. The final excel files must be uploaded to the blackboard (assignment section) before the deadline. 3. Grading will be based on blackboard submission only. Please make sure you make well organized tables in excel as well. Put proper names, symbols, units etc. If anything is missing, there will be deduction of marks. 4. For any graph, there must be proper labelling and formatting. Problem-2 In a process producing KNO3 salt, 1500 kg/hr of a feed solution containing 15wt.% KNO3 is fed to an evaporator, which evaporates some water at 422K to produce 60 wt% KNO3 solution. This is then fed to a crystallizer at 311 K, where crystals containing 98 wt% KNO3 is removed as product. The saturated solution containing 45 wt% KNO3 is recycled to the evaporator. Conduct a material balance over the process to find out the flow rates of the streams of Product (P), water (W) and Recycle (R) in kg/hr. Water, (W) kg/hr Feed KNO3 15 wt% water 85wt.% Product (P) kg/hr Evaporator Crystallizer Recycle (R) kg/hr 45% KNO3 Figure 1 KNO3 production flow chart
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


