Question: 3 attempts left Check my work Using the equilibrium constant expression Kea [NHz] [N2][12] and the equilibrium constant of Keq -0.586, determine the equilibrium concentration
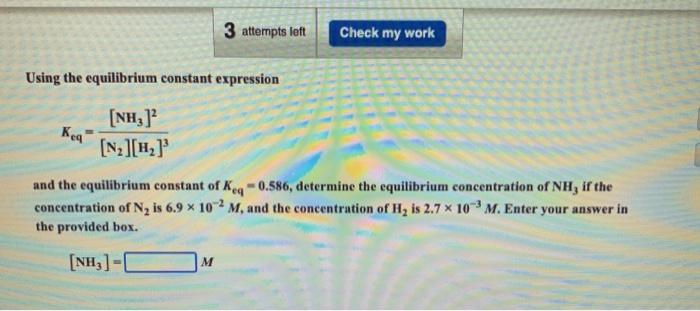
3 attempts left Check my work Using the equilibrium constant expression Kea [NHz] [N2][12] and the equilibrium constant of Keq -0.586, determine the equilibrium concentration of NH, if the concentration of N, is 6.9 x 102 M, and the concentration of H, is 2.7 10 M. Enter your answer in the provided box. (NH3)-1 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


