Question: 3. Breaking the Bond(s) A simplified model represents a hydrogen bond as the electrostatic interaction of four point charges arranged along a straight line, as
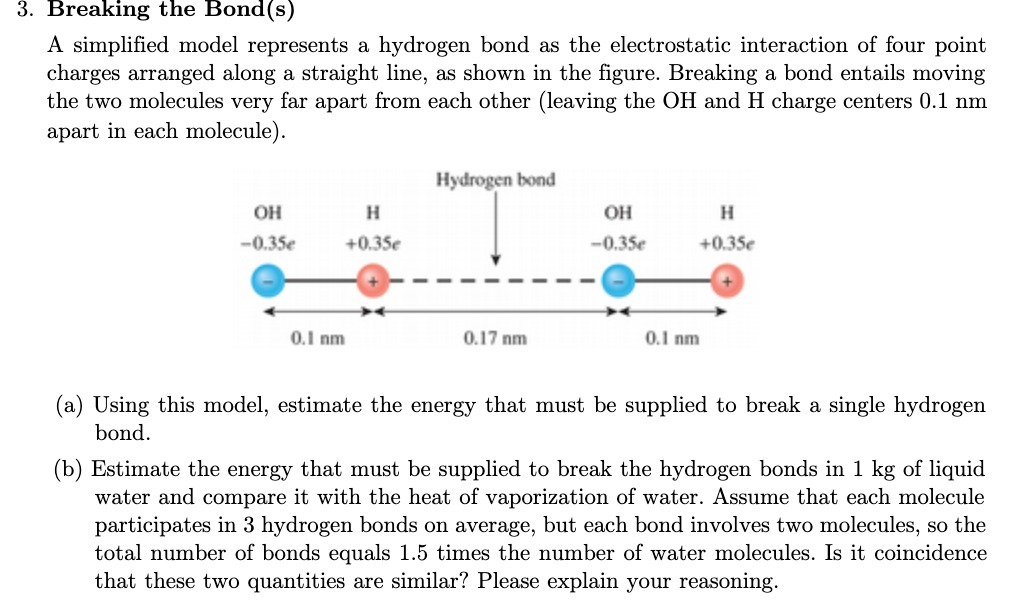
3. Breaking the Bond(s) A simplified model represents a hydrogen bond as the electrostatic interaction of four point charges arranged along a straight line, as shown in the figure. Breaking a bond entails moving the two molecules very far apart from each other (leaving the OH and H charge centers 0.1 nm apart in each molecule). Hydrogen bond OH H OH H -0.35e +0.35e -0.35e +0.35e 0.I nm 0.17 nm 0.1 nm (a) Using this model, estimate the energy that must be supplied to break a single hydrogen bond. (b) Estimate the energy that must be supplied to break the hydrogen bonds in 1 kg of liquid water and compare it with the heat of vaporization of water. Assume that each molecule participates in 3 hydrogen bonds on average, but each bond involves two molecules, so the total number of bonds equals 1.5 times the number of water molecules. Is it coincidence that these two quantities are similar? Please explain your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


