Question: 3. Consider a second-order reaction 2AB+C, which is occurred in 20 meters of 121 schedule 40 pipe packed with catalyst. There is 104.4lbm/h of gas
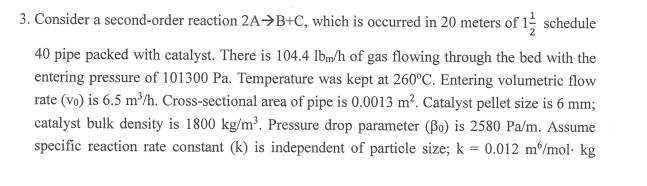

3. Consider a second-order reaction 2AB+C, which is occurred in 20 meters of 121 schedule 40 pipe packed with catalyst. There is 104.4lbm/h of gas flowing through the bed with the entering pressure of 101300Pa. Temperature was kept at 260C. Entering volumetric flow rate (v0) is 6.5m3/h. Cross-sectional area of pipe is 0.0013m2. Catalyst pellet size is 6mm; catalyst bulk density is 1800kg/m3. Pressure drop parameter (0) is 2580Pa/m. Assume specific reaction rate constant (k) is independent of particle size; k=0.012m6/molkg cat. h. Calculate the conversion with and without pressure drop. (20\%) If the catalyst size increases by a factor of 2 , what is the conversion with pressure drop? (10%)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


