Question: 3. Consider the surface-catalyzed oxidation of SO2 on solid urban particles. Let us assume that the reaction consists of SO2 absorption, followed by reaction of
3. Consider the surface-catalyzed oxidation of SO2 on solid urban particles. Let us assume that the reaction consists of SO2 absorption, followed by reaction of O2 and H2O to produce a molecule of H2SO4 adsorbed on the solid surface of the particles. The rate of the process is controlled by the rate of SO2 diffusion onto the particle surfaces and it stops when the particle surface is fully covered with H2SO4 molecules. Assume that each H2SO4 molecule occupies surface area of 30 A2 (1 A or Angstrom = 10-8 cm). Based on these assumptions, determine the total concentrations of sulfate (as g of S) that is possible under the following sets of conditions- note that the total volume of the particles is held constant, only their surface area changes. Which of these conditions (sets) produces a higher overall sulfuric acid concentration?
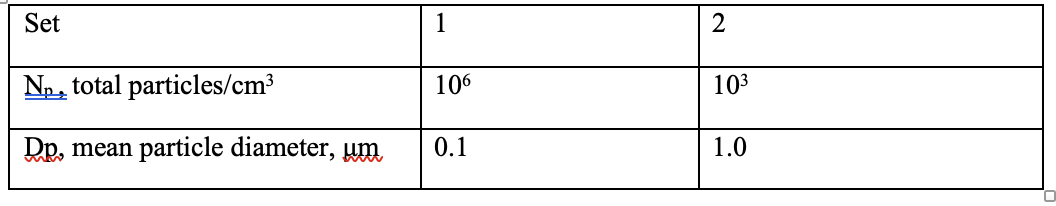
Set 1 2 No, total particles/cm3 106 103 Dp, mean particle diameter, um 0.1 1.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


